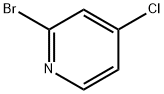
2-Bromo-4-chloropyridine synthesis
- Product Name:2-Bromo-4-chloropyridine
- CAS Number:22918-01-0
- Molecular formula:C5H3BrClN
- Molecular Weight:192.44

19798-80-2
527 suppliers
$6.00/1g

22918-01-0
263 suppliers
$6.00/1g
Yield:22918-01-0 92%
Reaction Conditions:
Stage #1:2-Amino-4-chloropyridine with hydrogen bromide;bromine in water at 0; for 0.166667 h;
Stage #2: with sodium nitrite in water at -10 - 20;
Stage #3: with sodium hydroxide in water; pH=> 10 at 0;
Steps:
8.g.1
To an aqueous solution of 48% strength hydrobromic acid (82 rnL) at 0 0C was added 4-chloro-2-pyridinamine (8.9 g, 69.2 mmol) followed by addition of bromine (33.4 g, 209 mmol) over 10 min. The resulting mixture was cooled to -10 0C and a solution OfNaNO2 (10.65 g, 154 mmol) in H2O (20 rnL) was poured in over a period of 30 min. The mixture was warmed at room temperature and stirred overnight. The mixture was recooled to 0 0C and NaOH (35%) added until the pH >10. The mixture was then extracted with ethyl acetate. The organic layer was then dried, filtered, and concentrated in vacuo. The product was purified via column chromatography (0-20% ethyl acetate/hexane) to afford 2-bromo-4-chloropyridine (12.1 g, 92%).
References:
SMITHKLINE BEECHAM CORPORATION;VITAE PHARMACEUTICALS, INC. WO2008/124575, 2008, A1 Location in patent:Page/Page column 110; 111
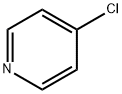
626-61-9
50 suppliers
$365.00/500mg

22918-01-0
263 suppliers
$6.00/1g
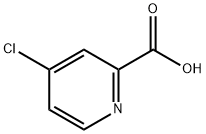
5470-22-4
312 suppliers
$7.00/5g

22918-01-0
263 suppliers
$6.00/1g