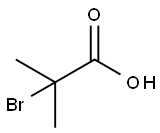
2-Bromo-2-methylpropionic acid synthesis
- Product Name:2-Bromo-2-methylpropionic acid
- CAS Number:2052-01-9
- Molecular formula:C4H7BrO2
- Molecular Weight:167
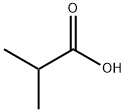
79-31-2
434 suppliers
$17.00/25mL
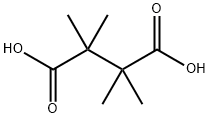
630-51-3
30 suppliers
$160.00/0.500g

2052-01-9
268 suppliers
$10.00/1g
Yield:630-51-3 66% ,2052-01-9 15%
Reaction Conditions:
Stage #1:isobutyric Acid with lithium diisopropyl amide in tetrahydrofuran at 0 - 40;Inert atmosphere;
Stage #2: with N,N-diethyl-N-bromoamine in tetrahydrofuran at 20 - 25; for 2 h;Inert atmosphere;
Steps:
Interaction of carboxylic acids with N,N-diethyl-N-haloamines
General procedure: A solution of 0.005 mol of carboxylic acid 1 in 20 mL of anhydrous THF was added atstirring to a solution of 0.01 mol of LDA in 30 mL of anhydrous THF cooled to 0-5° under argon atmosphere.The reaction mixture was warmed to 35-40°and stirred during 30-40 min. Then the mixture wascooled to 20-25°, and a solution of 0.005 mol ofN,N-diethyl-N-haloamine 3 in 20 mL of anhydrousTHF was added. The obtained mixture was stirredduring 2 h, and then 30 mL of distilled water was of HCl till pH 1, and the reaction products were extracted with diethyl ether (3×30 mL). Combinedextracts were dried over Na2SO4 and concentrated.After ether elimination, mixtures of dicarboxylic 8a-8c and α-halocarboxylic 9a-9f acids were obtained. Spectral parameters (1 and 13 NMR) of the products coincided with the reference data [2, 3].
References:
Zorin;Zainashev;Zorin [Russian Journal of General Chemistry,2016,vol. 86,# 11,p. 2469 - 2472][Zh. Obshch. Khim.,2016,vol. 86,# 11,p. 1826 - 1829,4]

79-31-2
434 suppliers
$17.00/25mL

2052-01-9
268 suppliers
$10.00/1g
19455-20-0
56 suppliers
$8.00/1g

2052-01-9
268 suppliers
$10.00/1g
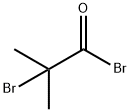
20769-85-1
262 suppliers
$13.00/5g
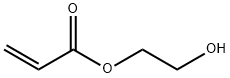
818-61-1
479 suppliers
$6.00/25g

2052-01-9
268 suppliers
$10.00/1g
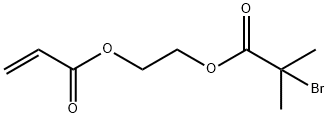
213453-02-2
1 suppliers
inquiry

20769-85-1
262 suppliers
$13.00/5g
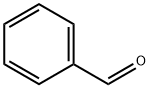
100-52-7
948 suppliers
$17.30/2g

2052-01-9
268 suppliers
$10.00/1g
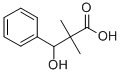
23985-59-3
5 suppliers
inquiry