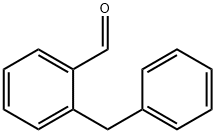
2-benzylbenzaldehyde synthesis
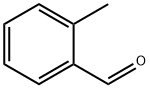
- Product Name:2-benzylbenzaldehyde
- CAS Number:32832-95-4
- Molecular formula:C14H12O
- Molecular Weight:196.24
Yield:32832-95-4 73%
Reaction Conditions:
with bis(acetato)bis(triphenylphosphine)palladium(0);glycinamide hydrochloride;silver trifluoroacetate;butyric acid in water at 20 - 120; for 48.5 h;Sealed tube;
Steps:
3. General procedure for the arylation of 2-methylbenzaldehydes
General procedure: A reaction tube (15 mL) with magnetic stir bar was charged with 2-methylbenzaldehyde (0.2 mmol), aryl iodide (0.3 mmol), silver trifluoroacetate (88.4 mg, 0.4 mmol), Pd(PPh3)2(OAc)2 (15.0 mg, 0.02 mmol), glycinamide hydrochloride (8.8 mg, 0.08 mmol), n-butyric acid (2 mL) and H2O (100 μL) in air. The reaction tube was sealed and allowed to stir at ambient temperature for 30 min, then heated to 120 °C for 48 h. Upon completion, the reaction mixture was cooled to room temperature, filtered through a silica gel plug, and the filtrate was diluted with dichloromethane and washed with saturated sodium carbonate solution. The resulting organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was isolated by column chromatography using petroleum ether and ethyl acetate or petroleum ether and dichloromethane as the eluent to give pure 2-benzylbenzaldehyde.
References:
Wen, Fei;Li, Zheng [Synthetic Communications,2020,vol. 50,# 22,p. 3462 - 3474] Location in patent:supporting information
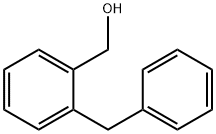
1586-00-1
31 suppliers
inquiry

32832-95-4
13 suppliers
inquiry
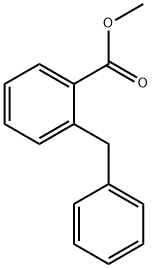
6962-60-3
7 suppliers
inquiry

32832-95-4
13 suppliers
inquiry