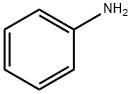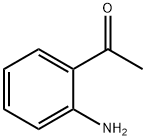
2-Aminoacetophenone synthesis
- Product Name:2-Aminoacetophenone
- CAS Number:551-93-9
- Molecular formula:C8H9NO
- Molecular Weight:135.16
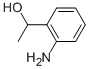
10517-50-7
52 suppliers
$31.00/1g

551-93-9
477 suppliers
$6.00/5g
Yield:551-93-9 98%
Reaction Conditions:
with N-chloro-N-(benzenesulfonyl)benzenesulfonamide;triethylamine in chloroform at 20 - 25; for 0.25 h;Inert atmosphere;chemoselective reaction;
Steps:
General procedure for oxidation of electron-rich alcohol and ether
General procedure: In a 3 neck, 50.0 mL round-bottomed flask arranged with a nitrogen balloon, calcium chloride guard tube, and magnetic stirrer was charged with chloroform 5.0 mL followed by PS-TEMPO, Biotage (0.6 g, 4.38 mol %). The reaction mixture was cooled to 20-25 °C and added NCBSI reagent portion I (0.29 mmol, 0.5 equiv. w. r. t PS-TEMPO) to activate the resin at once under a stream of nitrogen. The reaction mixture was stirred for 2 minutes. The colour change of resin was observed from yellow to dark red indicated the activation of resin. To the reaction mixture, alcohol or ether (13.1 mmol) was added followed by NCBSI reagent portion II (13.1 mmol). After addition, the colour of the resin changes from red to pale yellow which gives a preliminary indication of reaction completion. The reaction was monitored by TLC and confirm with 2,4-DNPH spray. On completion of the reaction, the TEMPO resin mixture was filtered and washed with chloroform. The filtrate was washed with 1% sodium bicarbonate solution, phase-separated, dried over anhyd. Na2SO4 and evaporated under reduced pressure to give the product. The purity of the product was determined by G.C.
References:
Badani, Purav;Chaturbhuj, Ganesh;Ganwir, Prerna;Misal, Balu;Palav, Amey [Tetrahedron Letters,2021,vol. 73] Location in patent:supporting information
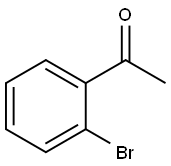
2142-69-0
319 suppliers
$6.00/1g

551-93-9
477 suppliers
$6.00/5g
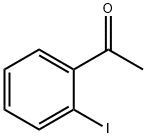
2142-70-3
163 suppliers
$6.00/1g

551-93-9
477 suppliers
$6.00/5g
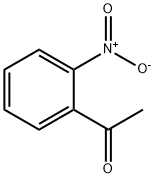
577-59-3
274 suppliers
$6.00/5g

551-93-9
477 suppliers
$6.00/5g