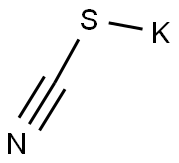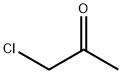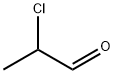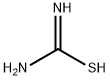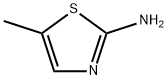
2-Amino-5-methylthiazole synthesis
- Product Name:2-Amino-5-methylthiazole
- CAS Number:7305-71-7
- Molecular formula:C4H6N2S
- Molecular Weight:114.17
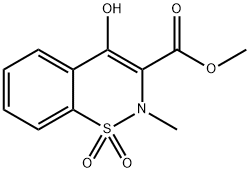
35511-15-0
181 suppliers
$75.00/5 g
71125-38-7
596 suppliers
$11.00/5g

7305-71-7
439 suppliers
$6.00/10g
Yield:7305-71-7 90%
Reaction Conditions:
with montmorillonite MK10 impregnated with ZnCl2 in o-xylene for 24 h;Reflux;Green chemistry;Reagent/catalyst;
Steps:
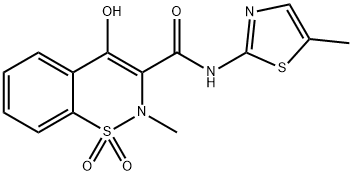
Synthesis of 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3-carbox-amide-1,1-di-oxyde (3) with impregnated MK10 (Zn-MK10) as catalyst
2-Amino-5-methylthiazole (85 mg, 0.7 mmol) and Zn-MK10 (30 mg, 0.037 mmol) were added to a solution of methyl 2-methyl-4-hydroxy-2H-1,2-benzothiazine-3-carboxylate 1,1-dioxide 4a (200 mg, 0.7 mmol) in o-xylene (3 mL) and the mixture was stirred at reflux during 24 h. After cooling to room temperature, the mixture was filtered to recover the supported catalyst, the xylene was evaporated and CH2Cl2 (10 mL) was added to the crude. The mixture was then purified on flash chromatography (EtOAc:n-heptane, 00:100 to 50:50) to provide the 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxyde (3) in 90% yield. White solid; mp 245-246 °C (CH2Cl2); Rf 0.62 (EtOAc:n-Heptane, 60:40), IR ν cm- 1: 3288, 1549, 1344, 1264, 1183 (Fig. 2). 1H NMR (400 MHz, CDCl3) δ (ppm): 2.46 (s, 3H, CH3), 2.89 (s, 3H, CH3), 7.23 (s, 1H, thiazolylH), 7.75 (dquint, J = 7.2, 1.6 Hz, 2H, ArH), 7.91 (dd, J = 7.4, 2.0 Hz, 1H, ArH), 8.06 (dd, J = 8.2, 1.6 Hz, 1H, ArH) (Fig. 3). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 11.8 (CH3), 38.0 (CH3), 114.3 (C), 123.3 (CH; C), 126.1 (CH), 129.3 (CH), 132.1 (CH), 132.9 (CH; C), 134.4 (2C), 155.7 (C), 168.4 (C) (Fig. 4).
References:
Dufrénoy, Pierrick;Ghinet, Alina;Hechelski, Marie;Da?ch, Adam;Waterlot, Christophe [Journal of the Iranian Chemical Society,2019,vol. 16,# 3,p. 501 - 509]
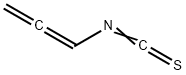
137768-73-1
0 suppliers
inquiry

7305-71-7
439 suppliers
$6.00/10g
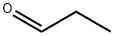
123-38-6
441 suppliers
$14.00/5mL

7305-71-7
439 suppliers
$6.00/10g