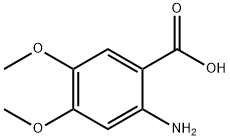
2-Amino-4,5-dimethoxybenzoic acid synthesis
- Product Name:2-Amino-4,5-dimethoxybenzoic acid
- CAS Number:5653-40-7
- Molecular formula:C9H11NO4
- Molecular Weight:197.19
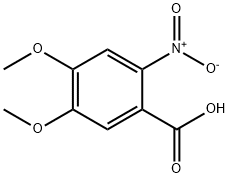
4998-07-6
221 suppliers
$6.00/5g

5653-40-7
278 suppliers
$12.50/5 g
Yield:5653-40-7 83%
Reaction Conditions:
Stage #1:4,5-dimethoxy-2-nitro-benzoic acid with hydrogen;palladium 10% on activated carbon in water; pH=6.6 at 50; under 2625.26 Torr;
Stage #2: with hydrogen;palladium 10% on activated carbon in water; pH=6.6 at 50; under 2625.26 Torr;
Steps:
1.A
48.13 g (0.729 mol) of KOH pellets (w=85%) are dissolved in 250 ml of ice water. 50 g (0.207 mol) methyl-4,5-dimethoxy-2-nitro-benzoate (1) are added to the clear solution and the resulting green suspension is heated to 70° C. During the heating a dark red solution is formed. Once the reaction has ended (monitored by HPLC) the solution is cooled to ambient temperature and adjusted to pH 6.6 with 34.6 g (0.570 mol) glacial acetic acid. The resulting red suspension is hydrogenated with 1 g of 10% Pd/C at 50° C. and 3.5 bar until the reaction comes to a standstill. Then the hydrogenation solution is filtered off and adjusted to pH 5.1 with 31.82 g (0.525 mol) glacial acetic acid under an inert gas. The light green suspension is stirred for 30 min at RT, then cooled to 5° C. and stirred for another 30 min.The product (2) is filtered off, washed in two batches with a total of 250 ml of ice water and then dried at 55° C. for 12 h in a vacuum drying cupboard.This reaction yielded 35.18 g (0.173 mol, 83% of theory) of light grey crystals.
References:
Jacobi, Albrecht;Schul, Michael US2008/319194, 2008, A1 Location in patent:Page/Page column 4
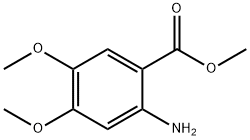
26759-46-6
207 suppliers
$6.00/5g

5653-40-7
278 suppliers
$12.50/5 g
93-07-2
555 suppliers
$5.00/5g

5653-40-7
278 suppliers
$12.50/5 g
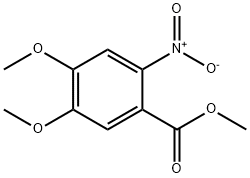
26791-93-5
147 suppliers
$10.00/1g

5653-40-7
278 suppliers
$12.50/5 g
2150-38-1
198 suppliers
$10.00/1g

5653-40-7
278 suppliers
$12.50/5 g