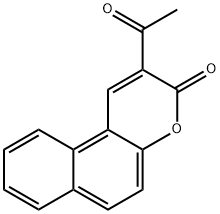
2-ACETYL-BENZO[F]CHROMEN-3-ONE synthesis
- Product Name:2-ACETYL-BENZO[F]CHROMEN-3-ONE
- CAS Number:727-80-0
- Molecular formula:C15H10O3
- Molecular Weight:238.24
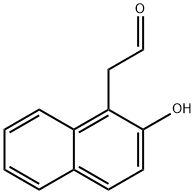
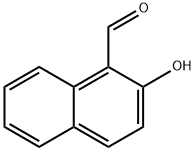
141-97-9
821 suppliers
$5.00/25g
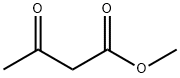
708-06-5
293 suppliers
$14.00/5g
![2-ACETYL-BENZO[F]CHROMEN-3-ONE](/CAS/GIF/727-80-0.gif)
727-80-0
16 suppliers
$22.00/500mg
Yield:727-80-0 97%
Reaction Conditions:
with piperidine in ethanol at 80; for 0.025 h;Microwave irradiation;Green chemistry;Knoevenagel Condensation;
Steps:
Synthesis of 3-Acetyl-2H-chromen-2-one (1a):
General procedure: A mixture salicylaldehyde S1 (2 mmol, 0.218 mL) and ethanol (20 mL) was stirred, and ethyl acetoacetate (2.4 mmol, 0.31 mL) was later added. Then, a catalytic amount of piperidine was added and swirled thoroughly. The reaction mixtures were added together in a microwave reaction vial and irradiated in microwave oven for a specific period of time and temperature as shown in Table S2 (Scheme S1). The reaction was monitored by TLC till completion. After completion, it was then allowed to cool for few minutes in iced water bath. A solid product was formed (Fig. S1). The product was ltered and dried to afford the product whose physicochemical properties are shown in Table S2. The same procedure was followed in the synthesis of other derivatives 1b-j. Each of the spectroscopic data is in agreement with the structures assigned.
References:
Yahaya, Issah;Sefero?lu, Nurgül;Sefero?lu, Zeynel [Tetrahedron,2019,vol. 75,# 14,p. 2143 - 2154] Location in patent:supporting information
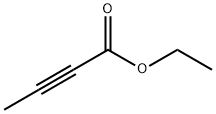
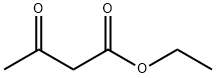
4341-76-8
231 suppliers
$10.00/1g

708-06-5
293 suppliers
$14.00/5g
![2-ACETYL-BENZO[F]CHROMEN-3-ONE](/CAS/GIF/727-80-0.gif)
727-80-0
16 suppliers
$22.00/500mg