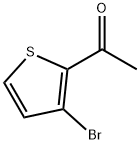
2-ACETYL-3-BROMOTHIOPHENE synthesis
- Product Name:2-ACETYL-3-BROMOTHIOPHENE
- CAS Number:42877-08-7
- Molecular formula:C6H5BrOS
- Molecular Weight:205.07

67-56-1
751 suppliers
$7.29/5ml-f
7311-64-0
228 suppliers
$7.00/1g

42877-08-7
100 suppliers
$8.00/250mg
Yield:-
Reaction Conditions:
with sulfuric acid for 24 h;Reflux;
Steps:
General procedures I for the synthesis of starting materials 1a-n
General procedure: 4.2.1. General procedures I for the synthesis of starting materials 1 a-n. Compounds 1a-g, i-n were synthesized according to general procedures I. Compound 1h was commercially available. Method A:16, 17 A two-necked round-bottomed flask equipped with a reflux condenser and a magnetic stir bar was charged withthe 2-bromobenzoic acid (20 mmol) and freshly distilled methanol(25 mL). The solution was heated in a hot water bath, conc. H2SO4(8 mmol) was added slowly and the reaction mixture was refluxed for 24 h. After cooling to room temperature around half of theamount of the solvent was removed in vacuo and the residue was partitioned between water (50 mL) and diethyl ether (70 mL). The organic layer was separated and washed with saturated NaHCO3(250 mL), water (50 mL) and brine (50 mL), dried over anhydrous MgSO4 and the volatiles were removed under reduced pressure.The crude product thus obtained was purified by flash chromatographyon silica gel to afford the alkyl-2-halobenzoate.In a two-necked round-bottomed flask equipped with a reflux condenser and a magnetic stir bar the alkyl 2-halobenzoate(22.5 mmol) was dissolved in freshly distilled dry THF (30 mL) under argon. The solution was cooled to 0 °C using an ice bath and NaH (60% in mineral oil, 15 mmol) was added portion wise. After stirring for 15 min a solution of the alkyl acetate (15 mmol) in dry THF (30 mL) was added dropwise to the reaction mixture at 0 °C.The mixture was warmed up, stirred at room temperature for 2 h and heated under reflux for 24 h. After cooling to room temperature around half of the amount of the solvent was removed in vacuo and the reaction mixture was diluted with toluene (50 mL). The resulting mixture was washed with 2N HCl (50 mL), saturated NH4Cl (50 mL), dried over anhydrous MgSO4 and the volatiles were removed under reduced pressure. The crude product was purifiedby flash column chromatography on silica gel to afford the alkyl 3-(20-halophenyl)-3-oxo-propanoate 1.
References:
Weischedel, Heike;Sudheendran, Kavitha;Mikhael, Alevtina;Conrad, Jürgen;Frey, Wolfgang;Beifuss, Uwe [Tetrahedron,2016,vol. 72,# 24,p. 3454 - 3467]
872-31-1
348 suppliers
$9.00/1g
75-36-5
578 suppliers
$17.92/100G

42877-08-7
100 suppliers
$8.00/250mg
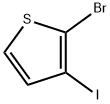
24287-92-1
25 suppliers
$498.23/5MG
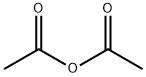
108-24-7
5 suppliers
$14.00/250ML

42877-08-7
100 suppliers
$8.00/250mg
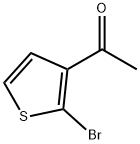
137272-68-5
21 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

42877-08-7
100 suppliers
$8.00/250mg

872-31-1
348 suppliers
$9.00/1g

108-24-7
5 suppliers
$14.00/250ML
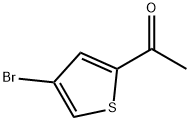
7209-11-2
110 suppliers
$16.00/250mg

42877-08-7
100 suppliers
$8.00/250mg