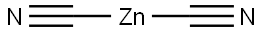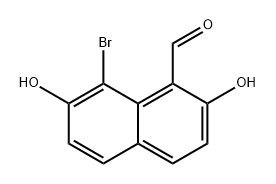
2,7-Dihydroxynaphthalene-1-carbaldehyde synthesis
- Product Name:2,7-Dihydroxynaphthalene-1-carbaldehyde
- CAS Number:20258-95-1
- Molecular formula:C11H8O3
- Molecular Weight:188.18
Yield:20258-95-1 78%
Reaction Conditions:
Stage #1: zinc(II) cyanide;2,7-Dihydroxynaphthalenewith hydrogenchloride in diethyl ether at 0 - 5;Gatterman Formylation;
Stage #2: with water in diethyl ether;ethanol;Cooling with ice;Heating;Gatterman Formylation;
Steps:
2,7-Dihydroxynaphthalene-1-carbaldehyde (2)
Anhydrous Zn(CN)2 (5.5g, 46.87 mmol) was taken in a three-necked round-bottomed flask and dry Et2O (120 mL) was added. The flask was then set-up on a magnetic stirrer maintaining the temperature between 0-5 °C under anhydrous conditions. 2,7-Dihydroxynaphthalene(5 g, 31.25 mmol) was then introduced into the flask along with dry Et2O (100 mL). Next, a stream of dry HCl gas was passed through the solution for ca. 4-5 h with continuous stirring. The solution gradually acquired a yellow color due to the intermediate product ‘a(chǎn)ldimine hydrochloride’. Further HCl gas was passed through the solution for 2-3 h. After the total consumption of 2,7-dihydroxynaphthalene (monitored by TLC), the entire mixture was poured slowly into ice cold H2O. EtOH (30 %) (100 mL) was added and then the suspension was heated on a water bath for a further 15-20 min until all the aldimine had dissolved. The solution was cooled on ice and a solid started separating slowly. The precipitated thick greenish solid was filtered using a Büchner funnel and washed several times with H2O. The obtained crude product was air-dried overnight and was crystallized by using absolute EtOH to afford a yellowish green crystal solid of the aldehyde derivative. Yield: 4.6 g (78 %); mp 158 °C (Lit.8 159-160 °C). IR (KBr): 3209, 2923, 1618, 1580, 1518, 1403, 1319, 1225, 761 cm-1. 1H NMR (300 MHz, CDCl3 + DMSO-d6): δ = 12.56 (s, 1 H, OH, D2O exch), 10.60 (s, 1 H, H-C=O), 9.80 (s, 1 H, OH), 7.817 (d, J = 9.0 Hz, 1 H,C-4), 7.812 (d, J = 9.3 Hz, 1 H, C-8), 7.55 (d, J = 8.7 Hz, 1 H, C-5), 6.90 (dd, J = 8.7 Hz, 2.1 Hz, 1 H, C-6), 6.79 (d, J = 9.0 Hz, 1 H, C-3). 13C NMR (75 MHz, CDCl3 + DMSO-d6): δ = 193.1, 165.1, 158.9, 138.9, 134.9, 130.9, 122.3, 116.2, 114.9, 110.9, 103.0. MS (EI, 70 eV): m/z (%) = 51 (13), 77 (28), 103 (20), 131 (43), 160 (65),188 (45).Anal. Calcd for C11H8O3: C, 70.21; H, 4.28. Found: C, 70.32; H, 4.14.
References:
Marathe, Suyog;Karnik, Anil V. [Synthesis,2016,vol. 48,# 24,art. no. SS-2016-Z0480-OP,p. 4589 - 4593]
51385-93-4
12 suppliers
$98.00/1g

20258-95-1
9 suppliers
inquiry
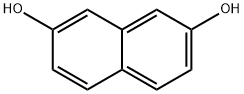
582-17-2
444 suppliers
$24.00/25g

20258-95-1
9 suppliers
inquiry
![2,7-Naphthalenediol, 1-[(phenylimino)methyl]-](/CAS/20210305/GIF/20258-94-0.gif)
20258-94-0
0 suppliers
inquiry

20258-95-1
9 suppliers
inquiry