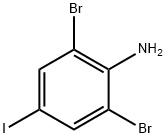
2,6-dibromo-4-iodoaniline synthesis
- Product Name:2,6-dibromo-4-iodoaniline
- CAS Number:10527-69-2
- Molecular formula:C6H4Br2IN
- Molecular Weight:376.82
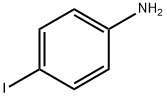
540-37-4
443 suppliers
$6.00/5g

10527-69-2
15 suppliers
inquiry
Yield:10527-69-2 95.5%
Reaction Conditions:
with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione in ethanol at 20 - 25; for 3.33333 h;
Steps:
1 Example 1:
The present invention is a method for preparing 3,5-dibromo iodobenzene comprising the steps of:Step one, 2,6-dibromo-4-iodoaniline intermediate step, is added in a container under iodoaniline and ethanol, normal temperature, was added portionwise DBDMH; HPLC detected no starting material spectrum when the product, the reaction was stopped; direct filtration to give 2,6-dibromo-4-iodoaniline intermediate; molar ratio of iodine aniline and DBDMH is 1: 1.Step two, in another vessel was added 2, 6-dibromo-4-iodoaniline intermediate and secondary phosphate, after cooling dripping Gaja nitrate solution, there will be a lot of solid washed out after completion of the dropping, direct filtration , recrystallized from absolute ethanol to give 3,5-dibromo iodobenzene needles.The molar ratio of 2,6-dibromo-4-iodoaniline intermediate and sodium nitrite is 1: 1.Preparation of intermediate 2,6-dibromo-4-iodo-aniline: Add in a container under iodoaniline and ethanol, normal temperature, was added portionwise DBDMH.When no starting material by HPLC, the reaction was stopped.Direct filtration to obtain the product.Detailed steps are as follows: where feeding amount as follows: iodine aniline: 219g, DBDMH: 285.9g, ethanol: 1800ml.219g in 2000ml reaction flask on iodoaniline (1mol) of anhydrous ethanol and 1000ml, stirred for 20 minutes, and dispersed uniformly.DBDMH was added portionwise 285.9g, the reaction temperature is controlled at 20-25 , about 2 hour period. After the addition of a large number of white solid precipitated.And then reacted at this temperature for 1 hour.The reaction was monitored by HPLC, when the raw material disappeared, the reaction was stopped, 20-25 filtration, 100 pressure and dried to obtain a white powder 360g.As shown in Table 1 and 2,6-dibromo-4-iodoaniline 1 shown in HPLC profiles, display profiles 2,6-dibromo-4-iodoaniline content of 99.34 percent, 95.5 percent yield of the reaction.
References:
CN105523884,2016,A Location in patent:Paragraph 0014; 0015; 0016
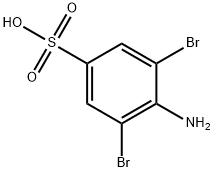
78824-10-9
12 suppliers
$28.60/250mg

10527-69-2
15 suppliers
inquiry

540-37-4
443 suppliers
$6.00/5g
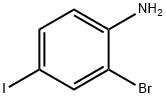
29632-73-3
104 suppliers
$7.00/250mg

10527-69-2
15 suppliers
inquiry
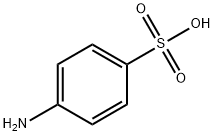
121-57-3
538 suppliers
$13.00/25g

10527-69-2
15 suppliers
inquiry

78824-10-9
12 suppliers
$28.60/250mg
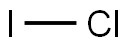
7790-99-0
253 suppliers
$48.39/25g
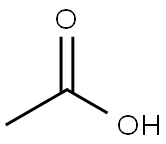
64-19-7
1559 suppliers
$10.00/25ML

10527-69-2
15 suppliers
inquiry