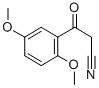
2,5-DIMETHOXYBENZOYLACETONITRILE synthesis
- Product Name:2,5-DIMETHOXYBENZOYLACETONITRILE
- CAS Number:898787-03-6
- Molecular formula:C11H11NO3
- Molecular Weight:205.21
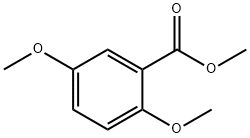
2150-40-5
50 suppliers
$22.00/250mg
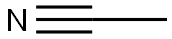
75-05-8
988 suppliers
$10.00/10g

898787-03-6
24 suppliers
inquiry
Yield:898787-03-6 40%
Reaction Conditions:
with sodium hydride in toluene;mineral oil at 0 - 110;
Steps:
iii.b 3-(2,5-Dimethoxyphenyl)-3-oxopropanenitrile I4
To a solution of methyl 2,5-dimethoxybenzoate I3 (4.5 g, 22.9 mmol) and acetonitrile (1.41 g, 16.0 mmol) in toluene (150 ml.) at 0 °C was added NaH (60% w/w dispersion in oil, 1.38 g, 34.4 mmol) and the mixture was stirred at 0 °C for 30 min then heated at 110 °C overnight. The reaction was quenched with water (400 ml.) and the mixture was extracted with EtOAc (300 ml. x 3). The combined organic extracts were washed with brine, dried over anhydrous Na2S04, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (petroleum ether/EtOAc = 30/1) to give the title compound I4 (1.9 g 40%) as a yellow solid. LCMS-A (ES-API): Rt 1.15 min; m/z 206.1 [M+H]+.
References:
WO2020/2587,2020,A1 Location in patent:Page/Page column 57
773837-37-9
0 suppliers
inquiry
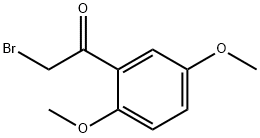
1204-21-3
145 suppliers
$16.00/250mg

898787-03-6
24 suppliers
inquiry
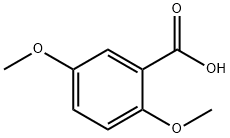
2785-98-0
213 suppliers
$40.00/5g

898787-03-6
24 suppliers
inquiry