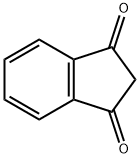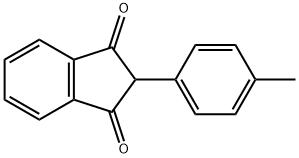
2-(4-METHYLPHENYL)-1H-INDENE-1,3(2H)-DIONE synthesis
- Product Name:2-(4-METHYLPHENYL)-1H-INDENE-1,3(2H)-DIONE
- CAS Number:7561-48-0
- Molecular formula:C16H12O2
- Molecular Weight:236.27
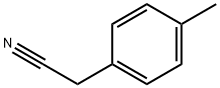
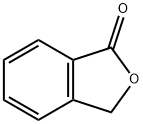
2947-61-7
267 suppliers
$8.00/5g
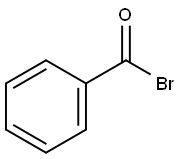
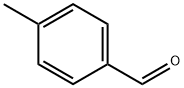
618-32-6
103 suppliers
$40.00/5g

7561-48-0
10 suppliers
$28.60/25MG
Yield:7561-48-0 96.2%
Reaction Conditions:
with 1,4-diaza-bicyclo[2.2.2]octane;bis(di-tert-?butyl(4-?dimethylaminophenyl)?phosphine)?dichloropalladium(II);trimethylphosphine(hexafluoroacetylacetonato)copper(I);1-butyl-3-methylimidazolium trifluoromethanesulfonimide salt;silver trifluoromethanesulfonate in ethylene glycol;dimethyl sulfoxide at 80; for 4 h;Inert atmosphere;Reagent/catalyst;
Steps:
1
At room temperature, the appropriate amount of organic solvent (DMSO and ethylene glycol as a mixture of equal volume), and (I) compound, 100mmol formula (II) compounds of the formula 100mmol, 12mmol catalyst (as 9.6mmol (A-taPhos)2PdCl2And a mixture of 2.4mmol trimethylphosphine (hexafluoroacetylacetonato) copper), 140mmol oxidant silver trifluoroacetate, 200mmol 10mmol base DABCO accelerator and 1-butyl-3-methylimidazolium trifluoromethane sulfonimide salt and then replaced with nitrogen several times, until the reaction atmosphere is a nitrogen atmosphere; temperature was elevated to 80 deg.] C, and stirred at this temperature for 4 hours; After completion of the reaction, the reaction solution was allowed to cool to room temperature, filtered, adjusted to neutral pH of the filtrate, water was sufficiently washed with saturated brine, and then ethyl acetate was added and extracted 3 times, the organic phases combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, the resulting residue was purified by column chromatography over silica gel 300-400 mesh, in a volume ratio of 1: 2 mixture of methylene chloride and acetone, elution was carried out to afford the compound of formula (III), in a yield of 96.2%.
References:
CN105461528,2016,A Location in patent:Paragraph 0035-0038
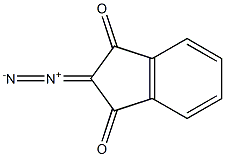
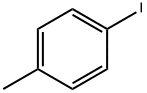
1807-49-4
0 suppliers
inquiry

108-88-3
0 suppliers
$14.00/100ml
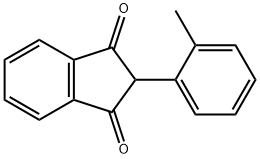
15432-97-0
8 suppliers
$31.00/250mg

7561-48-0
10 suppliers
$28.60/25MG
![1(3H)-Isobenzofuranone, 3-[(4-methylphenyl)methylene]-](/CAS/20210305/GIF/27695-13-2.gif)
27695-13-2
0 suppliers
inquiry

7561-48-0
10 suppliers
$28.60/25MG