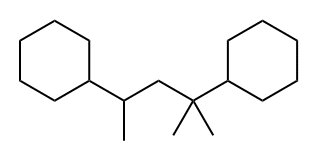
2,4-DICYCLOHEXYL-2-METHYLPENTANE synthesis
- Product Name:2,4-DICYCLOHEXYL-2-METHYLPENTANE
- CAS Number:38970-72-8
- Molecular formula:C18H34
- Molecular Weight:250.46
98-83-9
303 suppliers
$10.00/25mL

38970-72-8
18 suppliers
inquiry
Yield:38970-72-8 125 g
Reaction Conditions:
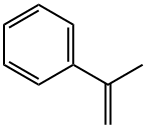
Stage #1: isopropenylbenzenewith 2-(2-methoxyethoxy)ethyl alcohol at 105; for 4 h;
Stage #2: with nickel at 250; under 23252.3 Torr; for 5 h;
Steps:
2 Manufacturing Example 2 Synthesis of Fluid 4
Manufacturing Example 2
Synthesis of Fluid 4
A hydrogenated linear dimer of α-methylstyrene was synthesized according to a method disclosed in International Publication WO2003/014268.
A 500-ml four-neck flask equipped with a reflux condenser, a stirring device and a thermometer was charged with 4 g of activated clay (Galleon Earth NS, manufactured by Mizusawa Industrial Chemical. Ltd.), 10 g of diethylene glycol monomethyl ether and 200 g of α-methylstyrene, and the mixture was heated at a reaction temperature of 105 degrees C. and stirred for four hours.
After finishing the reaction, the obtained liquid was analyzed by a gas chromatography to find that a conversion rate was 70%, a selectivity of the target product (a linear dimer of α-methylstyrene) was 95%, a selectivity of a byproduct (a cyclic dimer of α-methylstyrene) was 1%, and a selectivity of a substance having a high boiling point such as a trimer was 4%.
This reaction mixture was added into a one-liter autoclave with 15 g of a nickel/diatomaceous earth catalyst for hydrogenation (N-113, manufactured by Nikki Chemical Co., Ltd.) to carry out hydrogenation reaction (hydrogen pressure: 3 MPa·G, reaction temperature: 250 degrees C., reaction time: five hours).
The reactant was separated by filtration, concentrated and then distilled under reduced pressure to obtain 125 g of a hydrogenated linear dimer of α-methylstyrene (i.e., 2,4-dicyclohexyl-2-methylpentane) having a purity of 99% (Fluid 4).
References:
US2013/123553,2013,A1 Location in patent:Paragraph 0076; 0077