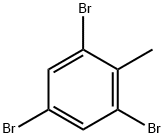
2,4,6-TRIBROMOTOLUENE synthesis
- Product Name:2,4,6-TRIBROMOTOLUENE
- CAS Number:6320-40-7
- Molecular formula:C7H5Br3
- Molecular Weight:328.83

108-88-3
7 suppliers
$21.80/5 mL
87-83-2
117 suppliers
$15.00/1g

6320-40-7
182 suppliers
$20.00/5 g
116779-85-2
2 suppliers
inquiry
93701-30-5
1 suppliers
inquiry
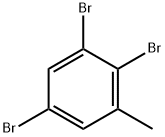
140141-58-8
2 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:toluene with bromine;aluminum (III) chloride in 1,2-dibromomethane at 25;
Stage #2: with sodium hydrogen sulfite;water at 45 - 65; for 1 h;Product distribution / selectivity;
Steps:
1
A 500 ml jacketed reactor provided with reflux condenser, thermowell, mechanical stirrer and toluene inlet, covered with heavy-duty aluminum foil against light penetration and connected to a heating cooling system was used. 150 ml of dry dibromomethane, DBM, 3.85gr, 0.0297 mole of aluminum chloride, AICI3, and 86 ml, 269.5 gr, 1.684 mole, of bromine, Br2 were introduced to the reactor. Temperature was set at 25°C and 33.4 ml, 28.9 gr, 0.314 mole, of toluene was fed via a peristaltic pump at a rate of 0.30 ml/min. The HBr generated was passed through two traps with DBM and a trap with solid CaCb to an absorption column in which water was recycled by means of a centrifugal pump.The conversion of toluene was followed-up by sampling the slurry of the reaction mixture, treating with water and sodium bisulphite solution, dissolving the solids in additional DBM and injecting the solution into a gas chromatograph. The reaction was almost finished when the feed of toluene was completed. A post reaction of one hour at 45°C - 65°C brought the reaction to completion.The pentabromotoluene, 5BT, content as determined by gas chromatograph was >99.5% and the sum of tribromotoluene, 3BT, all isomers, and tetrabromotoluene, 4BT, was less than 0.5%.Work-up and isolation of 5BT was done by adding water and sodium bisulphite solution to the reaction mixture for catalyst destruction and reduction of excess free bromine. The aqueous layer was separated and the organic slurry was washed with water, neutralized and filtered. The crystalline product was further dried in a vacuum oven. The product obtained had a melting point of 288°C - 289°C.
References:
BROMINE COMPOUNDS LTD. WO2006/8738, 2006, A1 Location in patent:Page/Page column 15-16
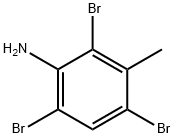
71642-16-5
37 suppliers
$23.39/5g

6320-40-7
182 suppliers
$20.00/5 g
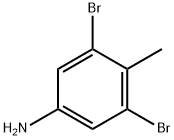
13194-73-5
110 suppliers
$22.00/250mg

6320-40-7
182 suppliers
$20.00/5 g
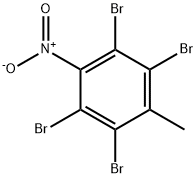
22230-45-1
0 suppliers
inquiry

6320-40-7
182 suppliers
$20.00/5 g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

6320-40-7
182 suppliers
$20.00/5 g