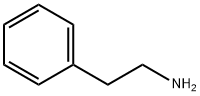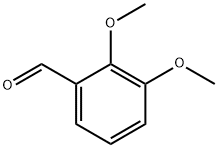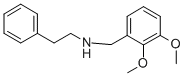
(2,3-DIMETHOXY-BENZYL)-PHENETHYL-AMINE synthesis
- Product Name:(2,3-DIMETHOXY-BENZYL)-PHENETHYL-AMINE
- CAS Number:101582-36-9
- Molecular formula:C17H21NO2
- Molecular Weight:271.35
![Benzeneethanamine, N-[(2,3-dimethoxyphenyl)methylene]-](/CAS/20210305/GIF/101584-62-7.gif)
101584-62-7
0 suppliers
inquiry

101582-36-9
12 suppliers
$378.00/1g
Yield:-
Reaction Conditions:
Stage #1: C17H19NO2with sodium tetrahydroborate in methanol; for 4 h;Reflux;
Stage #2: with hydrogenchloride in water;ethyl acetate; pH=5 - 6;
Steps:
5.1.1. General procedure for N-(2-arylethyl) isoquinolines analogs (7a-v)
General procedure: A 250 mL round bottom flask was charged with 2 (1.0 equiv) and 3 (1.0 equiv) at 70 °C, and the mixture was stirred under vacuum for 1 h. Methanol (100 mL) was added to the residue, and then NaBH4 (3.0 equiv) was added portionwise at the r.t. The mixture was refluxed for 4 h. Upon completion, as determined by TLC, the reaction mixture was concentrated in vacuo. The residue was dissolved in water (300 mL). The resulting aqueous phase was extracted with ethyl acetate (3 × 100 mL) and the combined organic layers were rinsed with saturated brines (100 mL) and dried (Na2SO4). Concentrated hydrochloric acid was added dropwise to adjust PH of the organic layer to 5-6, and filtered to give the hydrochlorate (5) without further purification.To a suspension of anhydrous CuSO4 (3.6 equiv) and 5 (1.0 equiv) in anhydrous formic acid (45 mL) was added glyoxal (40%, 2.0 equiv) at 100 °C. The reaction mixture was stirred at 100 °C for 5 h, concentrated hydrochloric acid (0.8 and 0.6 equiv) was added at 2 h and 4 h respectively during this process. Upon completion, as determined by TLC, the mixture was filtered. The filtrate was concentrated in vacuo. The residue (6) was dissolved in methanol (500 mL) and water (10 mL), CaO was added portionwise to adjust PH to 9-10. The suspension was stirred at the r.t. for 2 h, filtered and concentrated in vacuo. The resulting residues were purified via flash column chromatography using methanol/dichloromethane as the eluent, and then acidified by 10% HCl/ethanol to give 7.
References:
Wang, Yan-Xiang;Wang, Li;Xu, Yan-Ni;Li, Ying-Hong;Jiang, Jian-Dong;Si, Shu-Yi;Li, Yang-Biao;Ren, Gang;Shan, Yong-Qiang;Hong, Bin;Song, Dan-Qing [European Journal of Medicinal Chemistry,2011,vol. 46,# 4,p. 1066 - 1073] Location in patent:experimental part