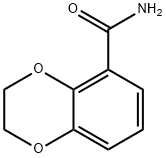
2,3-DIHYDRO-1,4-BENZODIOXINE-5-CARBOXAMIDE synthesis
- Product Name:2,3-DIHYDRO-1,4-BENZODIOXINE-5-CARBOXAMIDE
- CAS Number:349550-81-8
- Molecular formula:C9H9NO3
- Molecular Weight:179.17
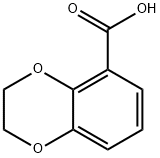
4442-53-9
179 suppliers
$7.00/1g

349550-81-8
43 suppliers
$24.00/100mg
Yield:349550-81-8 95%
Reaction Conditions:
Stage #1: 2,3-dihydrobenzo[1,4]dioxine-5-carboxylic acidwith 1,1′-carbonyldiimidazole in tetrahydrofuran at 20; for 1 h;
Stage #2: with ammonium hydroxide in tetrahydrofuran at 20; for 2 h;
Steps:
4.1.1.5. Procedure E: General procedure for the synthesis of the primary amides (K, a-v) from the respective carboxylic acids
General procedure: A mixture of theappropriate carboxylic acid (3 mmol, 1 equiv) and 1,1′ -carbonyldiimidazole(4.5 mmol, 1.5 equiv) in 30 ml anhydrous THF was stirredat room temperature for 1 h. This was followed by dropwise addition of15 ml 25% aqueous ammonia solution and stirring at room temperaturefor 2 h. Afterward, the mixture was diluted with water and extractedwith ethyl acetate (3 × 30 ml). To get rid of trace CDI and imidazole, thecombined organic layers were washed once either with 20 ml of 1 N HClor with 20 ml of 0.05 M potassium phosphate buffer pH 5.8 in case ofamides having basic heterocyclic rings (s-v). The organic fraction wasthen dried over anhydrous MgSO4 and evaporated under reduced pressureto give the pure primary amides in different yields, used directly innext steps without further purification.
References:
El-Gamil, Dalia S.;ElHady, Ahmed K.;Chen, Po-Jen;Hwang, Tsong-Long;Abadi, Ashraf H.;Abdel-Halim, Mohammad;Engel, Matthias [European Journal of Medicinal Chemistry,2022,vol. 238,art. no. 114411]
303-38-8
319 suppliers
$15.00/5g

349550-81-8
43 suppliers
$24.00/100mg
2411-83-8
103 suppliers
$13.00/1g

349550-81-8
43 suppliers
$24.00/100mg
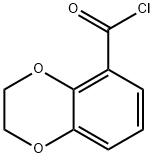
38871-41-9
52 suppliers
$45.00/25mg

349550-81-8
43 suppliers
$24.00/100mg