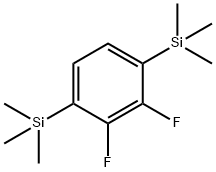
(2,3-difluoro-1,4-phenylene)bis(triMethylsilane) synthesis
- Product Name:(2,3-difluoro-1,4-phenylene)bis(triMethylsilane)
- CAS Number:867366-94-7
- Molecular formula:C12H20F2Si2
- Molecular Weight:258.45
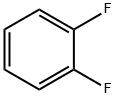
367-11-3
314 suppliers
$10.00/1g
108-18-9
365 suppliers
$10.00/1g

867366-94-7
14 suppliers
$956.12/5g
Yield:867366-94-7 118 g (100%)
Reaction Conditions:
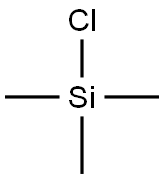
with n-butyllithium;chloro-trimethyl-silane;sulfuric acid in tetrahydrofuran;
Steps:
7 Succinic acid mono-{3-[3-(3-chloro-5-cyano-phenoxy)-2-fluoro-4-methoxy-benzyl]-4-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-ylmethyl}ester (56b)
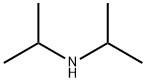
Example 7 Succinic acid mono-{3-[3-(3-chloro-5-cyano-phenoxy)-2-fluoro-4-methoxy-benzyl]-4-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-ylmethyl}ester (56b) See SCHEME 5 for steps 1 to 8. step 1-To a solution of di-iso-propylamine (150 mL, 108.3 g, 1.07 mol) in THF (500 mL) cooled to -78° C. and maintained under a N2 atmosphere was added over 1 15 min period, n-BuLi (100 mL, 1.00 mol, 10M in hexanes). The resulting mixture was stirred for 30 min at -78° C. A mixture of 23a (45 mL, 52.110 g, 0.457 mol) and chlorotrimethylsilane (130.0 mL, 111.28 g, 1.024 mol) was added at a rate which maintained the internal reaction temperature below -50° C. The solution was stirred at -78° C. for 1 h. The reaction was quenched at -78° C. by addition of 1M H2SO4, diluted with MTBE and the mixture was saturated with solid NaCl. The phases were separated and the aqueous phase was extracted with MTBE (300 mL). The combined organic extracts were dried (MgSO4), filtered and the solvents evaporated to afford 118 g (100%) of 23b as a white solid.
References:
US2011/207688,2011,A1