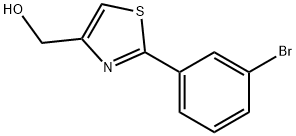
[2-(3-BROMO-PHENYL)-THIAZOL-4-YL]-METHANOL synthesis
- Product Name:[2-(3-BROMO-PHENYL)-THIAZOL-4-YL]-METHANOL
- CAS Number:885280-57-9
- Molecular formula:C10H8BrNOS
- Molecular Weight:270.15
786654-97-5
49 suppliers
$75.00/1g
![[2-(3-BROMO-PHENYL)-THIAZOL-4-YL]-METHANOL](/CAS/GIF/885280-57-9.gif)
885280-57-9
24 suppliers
$243.00/250mg
Yield:885280-57-9 79%
Reaction Conditions:
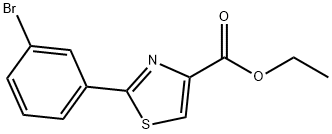
Stage #1: ethyl 2-(3-bromophenyl)-1,3-thiazole-4-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 0; for 0.583333 h;
Stage #2: with methanol;water in tetrahydrofuran;ethyl acetate;
Steps:
1.b
Example Ib; Preparation of [2- (bromo-phenyl) -thiazol-4-yl] -methanol; A solution of 2- (3-bromo-phenyl) -thiazole-4-carboxylic acid ethyl ester (6.0 g, 19.2 mmol) in THF (40 mL, 0.3 M) is cooled to 0 0C. Lithium aluminum hydride (LiAlH4) (1 M in THF; 17.2 mL, 17.2 mmol) is added dropwise over 5 minutes. After stirring for 30 minutes, the solution is diluted with ethyl acetate (40 mL) , saturated aq. NaCl (15 mL) and methanol (40 mL) . MgSO4 is added and the solution is filtered and concentrated. Purification by flash chromatography (15-25% ethyl acetate in heptane) gives [2- (bromo-phenyl) -thiazol-4- yl] -methanol (4.1 g, 79%) as a light yellow oil. 1H NMR (CDCl3, 300 MHz) δ 8.10 (t, J = 1.9 Hz, 1 H), 7.82 (dd, J1 = 1.9 Hz, J2 = 1.0 Hz, 1 H) , 7.53 (dd, J1 = 1.9 Hz, J2 = 1.0 Hz, 1 H), 7.29 (t, J = 7.9 Hz, 1 H), 7.20 (s, 1 H), 4.82 (d, J = 5.9 Hz, 2 H)
References:
WO2008/33934,2008,A1 Location in patent:Page/Page column 85-86