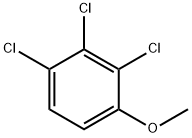
2,3,4-TRICHLOROANISOLE synthesis
- Product Name:2,3,4-TRICHLOROANISOLE
- CAS Number:54135-80-7
- Molecular formula:C7H5Cl3O
- Molecular Weight:211.47
634-66-2
124 suppliers
$20.89/100mg
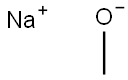
124-41-4
698 suppliers
$12.00/25g

74-88-4
346 suppliers
$15.00/10g

50375-10-5
38 suppliers
$24.89/25mg

54135-80-7
45 suppliers
$40.70/100mg-r
86607-59-2
1 suppliers
inquiry
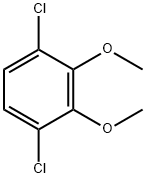
90283-02-6
1 suppliers
inquiry
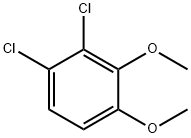
90283-00-4
1 suppliers
inquiry
Yield: 4% , 10% , 4%
Reaction Conditions:
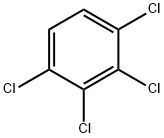
Stage #1:1,2,3,4,-tetrachlorobenzene;sodium methylate in N,N,N,N,N,N-hexamethylphosphoric triamide at 120; for 3 h;Inert atmosphere;
Stage #2:methyl iodide in N,N,N,N,N,N-hexamethylphosphoric triamide at 20; for 1 h;Inert atmosphere;
Steps:
General procedure for ipso-substitutions of chlorobenzenes to methoxybenzenes
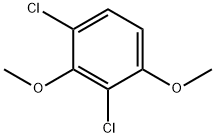
General procedure: According to the method of Testaferri et al. [4], sodium methoxide (4.0 equiv) was added to a stirred solution of the tetrachlorobenzene 11 or 12 (1.0 equiv) in hexamethylphosphoramide (0.3 M). The reaction mixture was stirred at 120 °C for 3 h and then cooled to room temperature. At this stage the mixture contains some minor amounts of phenols that are subsequently methylated by the addition of methyl iodide (1.5 equiv). The mixture was stirred for 1 h at room temperature and then poured into 2 M HCl. The aqueous phase was extracted three times with diethyl ether. The combined organic layers were dried over MgSO4 and concentrated in vacuo. Column chromatography on silica gel yielded the target compounds 10a, 10c, and 10e (from 11), and 10f and 10g (from 12). 1,2-Dichloro-3,4-dimethoxybenzene (10a), 1,4-dichloro-2,3-dimethoxybenzene (10c), and 1,3-dichloro-2,4-dimethoxybenzene (10e): The crude product contained mainly monosubstitution products, but small amounts of disubstitution products could be isolated by rigorous purification via column chromatography. The yields were: 10a (40 mg, 0.19 mmol, 4%, pale yellow oil), 10c (100 mg, 0.48 mmol, 10%, colourless solid), and 10e (40 mg, 0.19 mmol, 4%, colourless oil). 1,2-Dichloro-3,4-dimethoxybenzene (10a), 1,4-dichloro-2,3-dimethoxybenzene (10c), and 1,3-dichloro-2,4-dimethoxybenzene (10e): The crude product contained mainly monosubstitution products, but small amounts of disubstitution products could be isolated by rigorous purification via column chromatography. The yields were: 10a (40 mg, 0.19 mmol, 4%, pale yellow oil), 10c (100 mg, 0.48 mmol, 10%, colourless solid), and 10e (40 mg, 0.19 mmol, 4%, colourless oil).10a: TLC (hexane/ethyl acetate 5:1) Rf 0.25; IR (ATR) 3005 (w), 2939 (w), 2840 (w), 1579 (w), 1474 (m), 1431 (m), 1400 (m), 1291 (m), 1262 (m), 1169 (w), 1140 (w), 1043 (m), 1007 (m), 889 (w), 834 (m), 796 (m), 752 (w), 672 (m), 644 (w), 598 (w) cm-1; UV-vis (CH2Cl2) max (log ) 288 (3.23), 283 (3.22), 230 (3.84).10c: TLC (hexane/ethyl acetate 5:1) Rf 0.47; IR (ATR) 3003 (w), 2973 (w), 2941 (w), 2876 (w), 1579 (w), 1459 (m), 1430 (m), 1403 (m), 1239 (m), 1152 (w), 1128 (m), 1004 (s), 865 (m), 797 (m), 645 (m), 627 (m) cm-1; UV-vis (CH2Cl2) max (log ) 274 (2.60), 231 (3.87) nm.
References:
Wang, Tao;Rabe, Patrick;Citron, Christian A.;Dickschat, Jeroen S. [Beilstein Journal of Organic Chemistry,2013,vol. 9,p. 2767 - 2777] Location in patent:supporting information

634-66-2
124 suppliers
$20.89/100mg
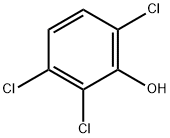
933-75-5
96 suppliers
$41.39/250mg

54135-80-7
45 suppliers
$40.70/100mg-r

634-66-2
124 suppliers
$20.89/100mg
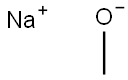
124-41-4
698 suppliers
$12.00/25g

50375-10-5
38 suppliers
$24.89/25mg

54135-80-7
45 suppliers
$40.70/100mg-r
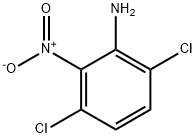
15944-74-8
5 suppliers
inquiry

54135-80-7
45 suppliers
$40.70/100mg-r
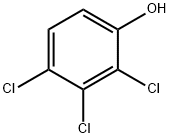
15950-66-0
72 suppliers
$37.60/48154
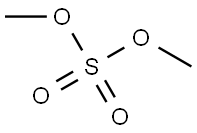
77-78-1
302 suppliers
$19.69/1ml

54135-80-7
45 suppliers
$40.70/100mg-r