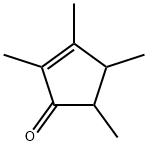
2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE synthesis
- Product Name:2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE
- CAS Number:54458-61-6
- Molecular formula:C9H14O
- Molecular Weight:138.21
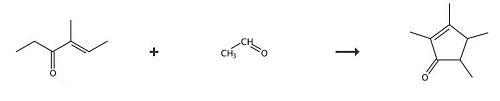
In a bottom round flask equipped with a mechanical stirrer, a dropping funnel and a reflux condenser was loaded 2000g (23.2 mol) of the starting ketone with 75% w/w of butylacetate as the solvent, 0.35 molar equivalents of anhydrous magnesium chloride and the aforementioned titanium catalytic solution containing 0.06 molar equivalents of the trichloropropoxytitanium complex. The resulting suspension was stirred vigorously and allowed to heat to 90°C. Then 2 molar equivalents of the acetaldehyde were added dropwise over 3h at 90°C. The reaction was continued for an additional hour and cooled to 40°C. The reaction mixture was hydrolysed with a 10% aqueous acetic acid solution and neutralised with a 20% aqueous potassium carbonate solution. The resulting organic phase was directly fractionated into a laboratory Sulzer packed column, to afford the title compound, as a mixture of isomers trans:cis = 85:15, in 27 % yield and the enone (II) (i.e. 4-methyl-4-hexen-3-one) in 31 % yield. mixture of isomers trans:cis = (B.p. = 70-80°C at P = 8 mbar); 4-methyl-4-hexen-3-one = (B.p. = 45-65°C at P = 8 mbar). 1H-NMR (isomer trans): 1.15 (d 3H); 1.19 (d 3H); 1.68 (s 3H); 1.88 (m 1H); 1.98 (s 3H); 2.25 (m 1H). 13C-NMR (isomer trans): 8.5; 14.6; 15.1 ; 17.7; 46.2; 48.4; 134.5; 171.6; 21 1.0.
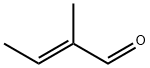
497-03-0
121 suppliers
$12.00/1g

54458-61-6
212 suppliers
$6.00/1g
Yield:-
Steps:
Multi-step reaction with 3 steps
2: MnO2 / pentane
3: HCO2H, H3PO4
References:
deVries,L. [Journal of Organic Chemistry,1960,vol. 25,p. 1838]
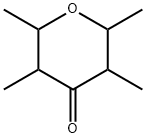
54458-60-5
4 suppliers
inquiry

54458-61-6
212 suppliers
$6.00/1g
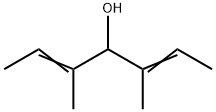
61005-41-2
0 suppliers
inquiry

54458-61-6
212 suppliers
$6.00/1g
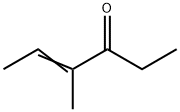
52883-78-0
0 suppliers
inquiry

54458-61-6
212 suppliers
$6.00/1g