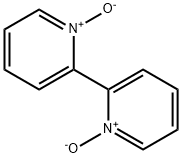
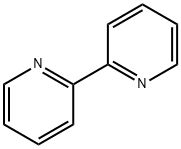
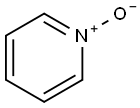
2,2'-Dipyridyl N-oxide synthesis
- Product Name:2,2'-Dipyridyl N-oxide
- CAS Number:33421-43-1
- Molecular formula:C10H8N2O
- Molecular Weight:172.18
Yield: 89.4%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in chloroform at 20; for 21.3 h;
Steps:
A solution of 2,2’-bipyridine(10.0 g, 64.0 mmol) in 38 mL ofCHCl3 was stirred at 0 °C for 35 min.A solution of m-chloroperbenzoic acid (21.6 g, 86.5 mmol, purity 69.0 - 75.0%) in 150mL of CHCl3 was added dropwise to the mixture over 80 min and the mixture wasstirred at room temperature for 20 h. The solution was washed with 5% aqueousNa2CO3 solution (100 mL × 3), dried over anhydrous Na2SO4, and concentrated. TheNa2CO3 washings were extracted with CHCl3 (70 mL × 4). The extract was dried overanhydrous Na2SO4 and concentrated. Each residual oil was put together, then themixture was dissolved in ether and undissolved 2,2’-dioxide was removed by filtration.The filtrate was concentrated and the residue was dried under vacuum to obtaingray-brown 2,2’-bipyridine N-oxide (9.85 g, 57.2 mmol, 89.4%).1H NMR (CDCl3): = 8.90 (dd, J1 = 8.1 Hz, J2 = 1.1 Hz, 1H), 8.74 (dd, J1 = 4.2 Hz, J2= 1.1 Hz, 1H), 8.32 (dd, J1 = 6.8 Hz, J2 = 2.2 Hz, 1H), 7.84 (ddd, J1 = 8.1 Hz, J2 = 7.8 Hz, J3 = 1.8 Hz, 1H), 7.40-7.33 (m, 2H), 7.27 (ddd, J1 = 7.4 Hz, J2 = 6.8 Hz, J3 = 2.2 Hz,1H). IR (KBr): = 3394, 3060, 1582, 1463, 1442, 1415, 1250, 1033, 849, 768, 720, 617,575 cm-1. Mp: 56-57 °C.
References:
Kodama, Koichi;Kobayashi, Akinori;Hirose, Takuji [Tetrahedron Letters,2013,vol. 54,# 40,p. 5514 - 5517] Location in patent:supporting information
366-18-7
646 suppliers
$5.00/5g

33421-43-1
55 suppliers
$99.50/5g
694-59-7
288 suppliers
$7.00/5g

33421-43-1
55 suppliers
$99.50/5g
14305-17-0
94 suppliers
$90.00/50mg
13737-05-8
37 suppliers
$155.00/1g

33421-43-1
55 suppliers
$99.50/5g