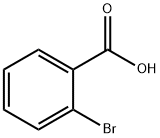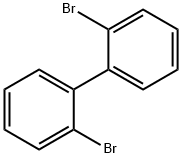
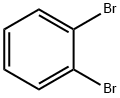
2,2'-Dibromobiphenyl synthesis
- Product Name:2,2'-Dibromobiphenyl
- CAS Number:13029-09-9
- Molecular formula:C12H8Br2
- Molecular Weight:312
583-53-9
314 suppliers
$10.00/5g

13029-09-9
285 suppliers
$20.00/1g
Yield:13029-09-9 84%
Reaction Conditions:
Stage #1:2,3-dibromobenzene with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;hexane;water at 0;Inert atmosphere;
Steps:
2,2'-Dibromobiphenyl (1a)
6.25 mL n-BuLi (10 mmol, 1.6 M in n-hexane) was added dropwise to a solution of4.72 g (20 mmol) of o-dibromobenzene in THF (50 mL) at -78 °C and stirred for 30min. After addition, the mixture was warmed to 0 °C and subsequently hydrolyzedwith aqueous HCl (10 mL, 3 N). The organic solvents were removed by rotaryevaporation, and the residue was extracted with diethyl ether. The combined filtrateswere concentrated under reduced pressure and the crude product was purified byrecrystallization in hexane to give 2.59 g pure 2,2'-dibromobiphenyl in 84% yield. 1HNMR (400 MHz, CDCl3) δ 7.66-7.68 (m, 2H), 7.38-7.40 (m, 2H), 7.23-7.29 (m, 4H).
References:
Ma, Jun;Li, Gaoqiang;Qiao, Yan;Tu, Jingxuan;Liu, Sha;Xu, Feng [Synlett,2015,vol. 26,# 14,art. no. ST-2015-W0198-L,p. 1991 - 1996] Location in patent:supporting information
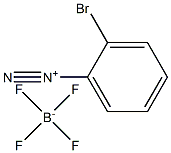
10448-07-4
0 suppliers
inquiry

13029-09-9
285 suppliers
$20.00/1g
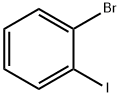
583-55-1
468 suppliers
$7.00/5g

13029-09-9
285 suppliers
$20.00/1g
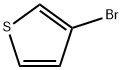
872-31-1
348 suppliers
$9.00/1g

583-53-9
314 suppliers
$10.00/5g

13029-09-9
285 suppliers
$20.00/1g
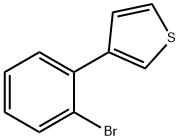
20608-83-7
9 suppliers
inquiry