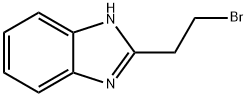
2-(2-Bromoethyl)benzoimidazole synthesis
- Product Name:2-(2-Bromoethyl)benzoimidazole
- CAS Number:4078-54-0
- Molecular formula:C9H9BrN2
- Molecular Weight:225.09
Yield:-
Reaction Conditions:
with hydrogenchloride in water; for 6 h;Reflux;
Steps:
Synthesis 2-(1H-benzo[d]imidazol-2-yl)ethyl (pyridin-3-ylmethyl) carbamodithio-ate (17l)
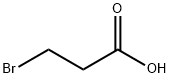
A mixture of 1,2-diaminobenzene (0.22g, 2mmol) and 3-bromopropionic acid (0.61g, 4mmol) in concentrated HCl (15mL) was refluxed for 6h. After cooling to room temperature, the mixture was adjusted to pH=7 with sodium bicarbonate, then extacted with ethyl acetate (20mL×3). The organic layer was combined and concentrated to afford crude 2-(2-bromoethyl)-1H-benzo[d]imidazole as a yellow oil. This crude product was used without purification. (0049) Following the same procedure for the synthesis of 17a, target compound 17l was obtained using 2-(2-bromoethyl)-1H-benzo[d]imidazole as halide. Yield 33%. White solid. MP: 149-151°C. 1H NMR (400MHz, DMSO-d6): δ=3.20-3.24 (t, J=6.8Hz, 2H), 3.67-3.71 (t, J=6.8Hz, 2H), 4.85 (d, J=5.2Hz, 2H), 7.13-7.15 (m, 2H), 7.35-7.38 (m, 1H), 7.48-7.50 (m, 2H), 7.68-7.70 (m, 1H), 8.47-8.52 (m, 2H), 10.54 (bs, 1H); 13C NMR (100MHz, DMSO-d6): δ=23.71, 29.00, 47.56, 122.02, 123.98, 148.88, 149.52, 153.44, 197.41; Anal. Cald for C16H16N4S2: C, 58.51; H, 4.91; N, 17.06; Found: C, 58.64; H, 4.58; N, 16.69.
References:
Li, Ying-Bo;Yan, Xu;Li, Ri-Dong;Liu, Peng;Sun, Shao-Qian;Wang, Xin;Cui, Jing-Rong;Zhou, De-Min;Ge, Ze-Mei;Li, Run-Tao [European Journal of Medicinal Chemistry,2016,vol. 112,p. 217 - 230]
51036-80-7
15 suppliers
inquiry

4078-54-0
30 suppliers
inquiry
95-54-5
516 suppliers
$9.00/1g

4078-54-0
30 suppliers
inquiry