Yield:-
Reaction Conditions:
with potassium carbonate in dichloromethane at 20; for 0.5 h;Product distribution / selectivity;
Steps:
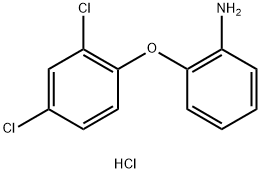
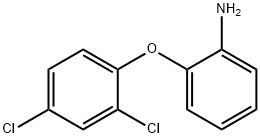
2-(2,4-Dichloro-phenoxy)-phenylamine hydrochloride (135 mg, 0.47 mmol) was dissolved in DCM (10 ml) and .to this was added K2CO3 (128 mg, 0.94 mmol) the reaction was then stirred at r.t. for 30 min. H2O was added to the reaction and the mixture was extracted with DCM. The organic layers were combined, dried (MgSO4) filtered and evaporated in vacuo. The resulting free amine was dissolved in DCE (2 ml) and to this was added N-(2-formyl-4,5-dimethoxy-phenyl)-acetamide (69 mg, 0.31 mmol) acetic acid (0.12 ml) and sodium triacetoxyborohydride (164 mg, 0.8 mmol). The resulting reaction mixture was stirred at r.t. for 2 h. NaHCO3 was then added, and repeatedly extracted with DCM. The organic layers were combined, dried (MgSO4), filtered and evaporated in vacuo. The crude mixture was purified using flash chromatography (0-50 % EtOAc in hexane) to afford the title compound as an off-white solid, 55 mg, 38 % yield. R.f. 0.58 (EtOAc), m.p. 128-131 °C, LCMS: tr = 1.1 min (95 % MeOH in water), m/z M-H 459.24, HPLC: U= 2.2 min (90 % ACN in H2O), 96 %, 1H NMR (CDCl3, 270 MHz,): δ 1.98 (3H, s, CH3), 3.83 (3H, s, OCH3), 3.87 (3H, s, OCH3), 4.26 (2H, s, CH2), 4.34 (IH, br.s, NH), 6.75-6.77 (3H, m, ArH), 6.83-6.91 (2H, m, ArH), 7.02-7.11 (IH, m, ArH), 7.15 (IH, dd, J= 1.7, 8.7Hz, ArH), 7.61 (IH, s, ArH), 8.31 (IH, br.s, NHCO). 13C NMR (CDCl3, 101 MHz): δ 24.5 (CH3), 47.2 (CH2), 56.1, 56.3 (OCH3), 104.0 (ArC)3 107.4, 112.5, 113.6, 118.0, 119.1, 120.0, 125.4 (ArCH), 125.5, 125.8 (ArC), 128.1 (ArCH), 129.0 (ArC), 130.6 (ArCH), 139.1, 143.7, 146.0, 148.7, 151.5 (ArC), 168.5 (CO).HRMS: Calcd for C23H22Cl2N2O4 (M+H)+ 461.1029, found (M+H)+ 461.1028.Synthesis of N-(4-chloro-2-[2-(2,4-dichloro-phenoxy)-phenylamino]-methyl-phenyl)- acetamide, C2IH17Cl3N2O2, MW 435.73,2-(2,4-Dichloro-phenoxy)-phenylamine hydrochloride (350 mg, 1.22 mmol) was dissolved in DCM (10 ml) and to this was added K2CO3 (335 mg, 2.44 mmol), the reaction was then stirred at r.t. for 30 min. H2O was then added and the mixture was extracted with DCM. The organic layers were combined, dried (MgSO4), filtered and evaporated in vacuo. The resulting amine was dissolved in DCE (2 ml) and to this was added N-(4-chloro-2-formyl-phenyl)-acetamide (160 mg, 0.81 mmol), acetic acid (0.15 ml) and sodium triacetoxyborohydride (0.43 g, 2.02 mmol). The resulting reaction mixture was stirred at r.t. for 2 h. NaHCO3 was then added and the mixture was repeatedly extracted with DCM. The organic layers were combined, dried (MgSO4), filtered and evaporated in vacuo. The crude mixture was purified using flash chromatography (0-50% EtOAc in hexane) to afford the title compound as a cream solid, 210 mg, 60 % yield. R.f. 0.38 (EtOAc), m.p. 135-137 °C,LCMS: U= 1.3 min (95 % MeOH in water), m/z M-H 433.1, 435.1, HPLC: U= 2.8 min (90 % ACN in H2O), 99 %, 1H NMR (CDCl3, 270 MHz,): δ 1.98 (3H, s, CH3), 4.28-4.29 (2H, m, CH2), 4.40 (IH, br.s, NH), 6.70-6.88 (4H, m, ArH), 7.04-7.10 (IH, m, ArH), 7.17 (IH, dd, J = 2.5 Hz, ArH), 7.25-7.27 (2H, m, ArH), 7.45 (IH, d, J= 2.5 Hz, ArH), 7.96-7.99 (IH, m, ArCH), 8.64 (IH, br.s. NH), 13C NMR (CDCl3, 101 MHz): δ 24.6 (CH3), 47.4 (CH2), 113.8, 117.8, 119.7, 120.3, 124.1, 125.3 (ArCH), 125.9 (ArC), 128.2, 128.7, 129.3 (ArCH), 129.4, 129.6 (ArC), 130.7 (ArCH), 136.0, 138.6, 144.4, 151.1 (ArC), 168.5 (CO). HRMS: Calcd for C21H17Cl3N2O2 (M+H)+ 435.0428, found (M+H)+ 437.0387.Synthesis of N-(4-([2-(2,4-dicUoro-phenoxy)-phenylamino]-methyl)-phenyl)-acetamide, C21H18Cl2N2O2, MW 401.29,To a solution of 2-(2,4-dichloro-phenoxy)-phenylamine hydrochloride (0.15g, 0.52mmol) in DCM (10ml) was added K2CO3 (0.22g, 1.04mmol), the resulting solution was stirred at r.t for 30 min. Water was then added and the mixture was extracted with DCM. The organic layers were combined, dried (MgSO4), filtered and evaporated in vacuo. The resulting amine was dissolved in DCE (3ml) and to this was added 4- acetamidobenzaldehyde (0.126 g, 0.0.75mmol), acetic acid (0.11 ml) and sodium triacetoxyborohydride (0.27g, 1.3mmol). The resulting reaction mixture was stirred at r.t. for 2h. NaHCO3 was then added and the mixture was repeatedly extracted with DCM. The organic layers were combined, dried (MgSO4), filtered and evaporated in vacuo. The crude mixture was purified using flash chromatography (0-100 % EtOAc in hexane) to afford the title compound as a white waxy solid, 142 mg, 69 % yield. R.f. 5.8 (EtOAc),LCMS: tr= 1.2 min (95 % MeOH in H2O), m/z M-H 399.03, 401.04,HPLC: tτ= 2A2 min (90 % ACN in H2O), 98 %,1H NMR (CDCl3, 270 MHz): δ 2.15 (3H, s, CH3), 4.31 (2H, s, CH2), 4.59 (IH, br.s, NH),6.59-6.68 (2H, m, ArH), 6.75 (IH, dd, J = 1.5, 7.9Hz, ArH), 6.80 (IH, d, J = 8.7 Hz, ArH), 6.96-7.02 (IH, m, ArH), 7.12 (IH, dd, J = 2.5, 8.9 Hz, ArH), 7.22-7.31 (IH, m, ArH), 7.41-7.45 (2H, m, ArH).13C NMR (CDCl3, 68 MHz): δ 24.7 (CH3), 47.3 (CH2), 112.2, 117.1, 118.5, 119.4, 120.2 (ArCH), 125.3 (ArC), 125.5, 127.9, 128.0 (ArCH), 128.2 (ArC), 130.4 (ArCH), 135.1, 136.9, 139.7, 142.7, 151.8 (ArC), 168.4 (CO). HRMS: Calcd for C2IH18Cl2N2O2 (M+H)+ 399.0673, found (M+H)+ 399.0674. Anal, calcd for C21H18Cl2N2O2 C 62.85, H 4.52 N 6.98 %. Found: C 62.7, H 4.52, N 6.92 %.
References:
WO2009/66072,2009,A2 Location in patent:Page/Page column 65; 72; 82