1H-PYRROLO[3,2-C]PYRIDINE-2-CARBALDEHYDE synthesis
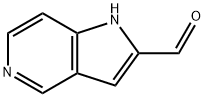
- Product Name:1H-PYRROLO[3,2-C]PYRIDINE-2-CARBALDEHYDE
- CAS Number:630395-95-8
- Molecular formula:C8H6N2O
- Molecular Weight:146.15
![tert-butyl 2-(diethoxymethyl)-1H-pyrrolo[3,2-c]pyridine-1-carboxylate](/CAS/20180601/GIF/853685-76-4.gif)
853685-76-4
6 suppliers
inquiry
![1H-PYRROLO[3,2-C]PYRIDINE-2-CARBALDEHYDE](/CAS/GIF/630395-95-8.gif)
630395-95-8
93 suppliers
$87.00/100mg
![2-Formyl-pyrrolo[3,2-c]pyridine-1-carboxylic acid tert-butyl ester](/CAS/20180601/GIF/853685-77-5.gif)
853685-77-5
1 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;water at 20; for 20 h;
Steps:
3.i 1H-Pyrrolo[3,2-c]pyridine-2-carboxaldehyde, 9b-1 (Scheme 3, step i)
1H-Pyrrolo[3,2-c]pyridine-2-carboxaldehyde, 9b-1 (Scheme 3, step i) Add to a solution of 2-(diethoxymethyl)-1H-pyrrolo[3,2-c]pyridine-1-carboxylic acid 1,1-dimethylethyl ester (8b-1, 9.8 g, 30.6 mmol) in 100 ml THF, 6 ml of concentrated HCl. Stir the reaction mixture at room temperature for 20 h, basify with saturated sodium bicarbonate solution, pour into EtOAc, wash with saturated sodium bicarbonate and brine, dry the organic phase over MgSO4, filter, and concentrate to give a mixture of 2-formyl-1H-pyrrolo[3,2-c]pyridine-1-carboxylic acid 1,1-dimethylethyl ester and 1H-pyrrolo[3,2-c]pyridine-2-carboxaldehyde 9b-1 as a solid. Separate the mixture by flash chromatography (silica, 1-3% MeOH/CH2Cl2) to give 2-formyl-1H-pyrrolo[3,2-c]pyridine-1-carboxylic acid 1,1-dimethylethyl ester (5.0 g) as an oil and 1H-pyrrolo[3,2-c]pyridine-2-carboxaldehyde 9b-1 (1.0 g) as a tan solid. Separate the mixture by flash chromatography (silica, 1-3% MeOH/CH2Cl2) to give 2-formyl-1H-pyrrolo[3,2-c]pyridine-1-carboxylic acid 1,1-dimethylethyl ester (5.0 g) as an oil and 1H-pyrrolo[3,2-c]pyridine-2-carboxaldehyde 9b-1 (1.0 g) as a tan solid. Add TFA (5.0 mL) dropwise to a solution of 2-formyl-1H-pyrrolo[3,2-c]pyridine-1-carboxylic acid 1,1-dimethylethyl ester (5.0 g, 20.3 mmol) in 250 ml dichloromethane. Heat the reaction mixture at reflux for 3 hours, concentrate, dilute the residue with 300 ml EtOAc, wash with saturated sodium bicarbonate solution (3*) and brine, dry the organic phase (MgSO4), filter and concentrate to yield 1H-pyrrolo[3,2-c]pyridine-2-carboxaldehyde 9b-1 (2.24 g) as a pure solid.
References:
US2005/131012,2005,A1 Location in patent:Page/Page column 11; 20
![2-Formyl-pyrrolo[3,2-c]pyridine-1-carboxylic acid tert-butyl ester](/CAS/20180601/GIF/853685-77-5.gif)
853685-77-5
1 suppliers
inquiry
![1H-PYRROLO[3,2-C]PYRIDINE-2-CARBALDEHYDE](/CAS/GIF/630395-95-8.gif)
630395-95-8
93 suppliers
$87.00/100mg