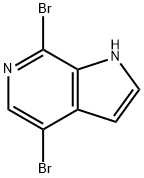
1H-Pyrrolo[2,3-c]pyridine, 4,7-dibroMo- synthesis
- Product Name:1H-Pyrrolo[2,3-c]pyridine, 4,7-dibroMo-
- CAS Number:619331-71-4
- Molecular formula:C7H4Br2N2
- Molecular Weight:275.93
![1H-Pyrrolo[2,3-c]pyridine, 4,7-dibroMo-](/CAS/GIF/619331-71-4.gif)
619331-71-4
35 suppliers
$75.00/50mg
![1H-Pyrrolo[2,3-c]pyridine-7-carbonitrile,4-methoxy-(9CI)](/CAS/GIF/619331-72-5.gif)
619331-72-5
5 suppliers
inquiry
Yield:619331-72-5 25%
Reaction Conditions:
Stage #1: 4,7-dibromo-1H-pyrrolo[2,3-c]pyridinewith copper(I) cyanide in DMF (N,N-dimethyl-formamide) at 150; for 1 h;
Stage #2: in methanol;DMF (N,N-dimethyl-formamide) at 110; for 0.166667 h;
Steps:
Preparation of Precursor 2v:
To a mixture of 2u (2.0 g, 7.3 mmol) and CuCN (1.0 g, 11 mmol) was added DMF (20 ml). The reaction mixture was heated at 150° C. for 1 hour. After cooling to room temperature, the reaction mixture was added NaOMe (20 ml, 25 wt. % solution in MeOH), and was heated at 110° C. for 10 minutes. After cooling to room temperature, the reaction mixture was poured into an aqueous solution of ammonium acetate (sat. 500 ml). The resulting mixture was filtered through a short Celite pad. The filtrate was extracted with EtOAc (500 ml×4). The combined extracts were dried over MgSO4 and evaporated in vacuo to give a brownish residue, which was triturated with MeOH (5 ml×3) to provide precursor 2v as a yellow solid (317 mg, 25%). The structure was supported by NOE experiments. 1H NMR: (DMSO-d6) 12.47 (s, 1H), 8.03 (s, 1H), 7.65 (t, J=2.8, 1H), 6.70 (dd, J=2.8, 1.8, 1H), 4.08 (s, 3H); LC/MS: (ES+) m/z (M+H)+=174; HPLC (alternate conditions B, column G) Rt=1.320.
References:
US2004/110785,2004,A1 Location in patent:Page 179-180