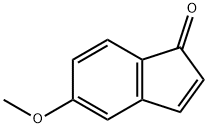
1H-INDEN-1-ONE, 5-METHOXY- synthesis
- Product Name:1H-INDEN-1-ONE, 5-METHOXY-
- CAS Number:72913-59-8
- Molecular formula:C10H8O2
- Molecular Weight:160.17
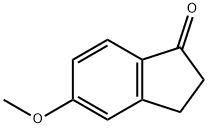
5111-70-6
233 suppliers
$34.00/5g

72913-59-8
12 suppliers
$100.00/100mg
Yield:72913-59-8 70%
Reaction Conditions:
Stage #1: 5-methoxy-1-indanonewith N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane; for 2.5 h;Reflux;
Stage #2: with triethylamine in tetrachloromethane at 0;
Steps:
Representative experimental procedure for the Synthesis of benzo[c]fluorenone
General procedure: NBS (195 mg, 1.1 mmol) and AIBN (2 mg, 0.01 mmol) were added to a CCl4 solution (4 ml) of the 6-methoxyindanone (162 mg, 1 mmol). The resulting mixture was stirred at reflux temperature for 2.5 h, then cooled and filtered through Celite, which was then washed with CCl4. The filtrate was cooled to 0 °C and treated with triethylamine (0.28 mL, 2.0 mmol) overnight, then concentrated in vacuuo. Chromatography of the residue (1 : 9~1 : 4 EtOAc/hexanes) afforded 97 mg (60%) of enone 1a as the pink oil which solidified as light red solid in refrigerator.
References:
Zheng, Shuyan;Tan, Hongsheng;Zhang, Xiaoguang;Yu, Chunhui;Shen, Zhengwu [Tetrahedron Letters,2014,vol. 55,# 5,p. 975 - 978] Location in patent:supporting information
79827-91-1
5 suppliers
inquiry

72913-59-8
12 suppliers
$100.00/100mg
51699-39-9
4 suppliers
inquiry

72913-59-8
12 suppliers
$100.00/100mg
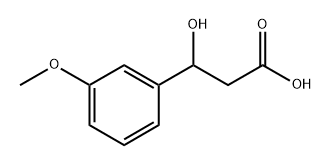
123208-85-5
1 suppliers
inquiry

72913-59-8
12 suppliers
$100.00/100mg
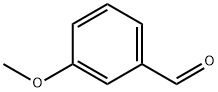
591-31-1
416 suppliers
$10.00/5g

72913-59-8
12 suppliers
$100.00/100mg