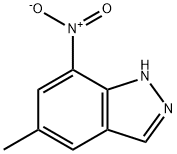
1H-Indazole, 5-Methyl-7-nitro- synthesis
- Product Name:1H-Indazole, 5-Methyl-7-nitro-
- CAS Number:113302-88-8
- Molecular formula:C8H7N3O2
- Molecular Weight:177.16
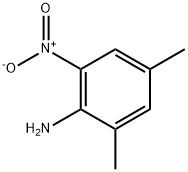
1635-84-3
129 suppliers
$5.00/100mg

113302-88-8
20 suppliers
inquiry
Yield:113302-88-8 36.6%
Reaction Conditions:
with acetic acid;sodium nitrite in water at 20; for 2 h;
Steps:
24.1 Step 1: Example 24b
To a solution of Example 24a (10.0 g, 60.24 mmol, 1.0 eq) in AcOH (150 mL) was added a solution of NaNO2 (4.99 g, 72.29 mmol, 1.2 eq) in H2O (10 mL) dropwise at r.t. The reaction mixture was stirred for 2 h at r.t. Then, water (100 mL) was added to the mixture, which was stirred for 30 min. The precipitated solid was collected by filtration, which was washed with H2O and MTBE. The crude product was purified by silica gel flash column chromatography to afford the desired product Example 24b (3.9 g, 36.6% yield) as a yellow solid. LCMS [M+1]+ = 178.2.
References:
WO2020/185755,2020,A1 Location in patent:Paragraph 00410
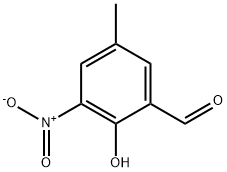
66620-31-3
7 suppliers
inquiry

113302-88-8
20 suppliers
inquiry
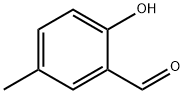
613-84-3
176 suppliers
$15.00/1g

113302-88-8
20 suppliers
inquiry
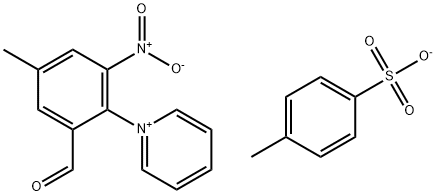
113302-82-2
0 suppliers
inquiry

113302-88-8
20 suppliers
inquiry
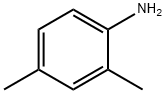
95-68-1
312 suppliers
$14.00/5g

113302-88-8
20 suppliers
inquiry