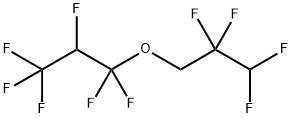
1H,1H,2'H,3H-DECAFLUORODIPROPYL ETHER synthesis
- Product Name:1H,1H,2'H,3H-DECAFLUORODIPROPYL ETHER
- CAS Number:65064-78-0
- Molecular formula:C6H4F10O
- Molecular Weight:282.08
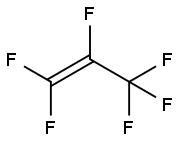
116-15-4
170 suppliers
$40.00/25 g
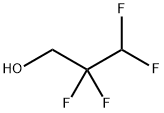
76-37-9
313 suppliers
$8.00/25g

65064-78-0
32 suppliers
$55.00/1 g
Yield:65064-78-0 99.8%
Reaction Conditions:
with potassium hydroxide in water at 50 - 60; under 3000.3 - 3750.38 Torr;Inert atmosphere;Autoclave;
Steps:
11
EXAMPLE 11; Step (A) The inside of a 3-liter stainless steel autoclave was evacuated, and after introducing potassium hydroxide (85 g: 1.5 mole), water (800 ml) and 2,2,3,3-tetrafluoropropyl alcohol (fluorine-containing alkyl alcohol): HCF2CF2CH2OH (600 g: 4.5 mole), evacuation and replacement with nitrogen gas of the inside of the autoclave were conducted three times at room temperature. After evacuation of the system, hexafluoropropylene (CF2=CFCF3) was introduced to give the inside pressure of the system of 0.1 MPa, and heating was conducted to give the inside temperature of 50°C. After the inside temperature had reached 50°C, hexafluoropropylene was added little by little so as to maintain the reaction pressure at 0.4 MPa to 0.5 MPa. The inside temperature of the system was adjusted so as to be maintained at 50°C to 60°C.Step (B) When the amount of added hexafluoropropylene reached 0.6 mole to 1 mole of fluorine-containing alkyl alcohol, supply of hexafluoropropylene was stopped and the reaction was continued with stirring. When lowering of the inside pressure of the autoclave stopped, the inside temperature of the autoclave was brought to room temperature and unreacted hexafluoropropylene was discharged to terminate the reaction. In the autoclave, a solution of reaction product separated into two layers of a lower organic layer (ether layer) and an upper water layer had been formed. After sampling the upper water layer, the amount of unreacted fluorine-containing alkyl alcohol determined by 19F-NMR was 7.1 mole. Whether or not impurities were present was checked, and neither potassium fluoride nor potassium difluoroacetate was detected.Step (C) Then, after washing the lower organic layer in the solution of reaction product with water once, the organic layer was separated and recovered. The amount of recovered organic layer was 761 g, and as a result of 19F-NMR and 1H-NMR analyses after washing with water once, the organic layer contained fluorine-containing ether represented by: [Show Image] and its purity measured by GC was 99.83 %. The amount of this produced fluorine-containing ether (761 g × 0.9983 = 760 g: 2.69 mole) was an amount corresponding to 60 % when converted to the conversion ratio of the fluorine-containing alkyl alcohol. At this stage, the yield was 59.8%.Step (D) Potassium hydroxide (basic compound) was not charged additionally.Step (E) From the amount of unreacted fluorine-containing alkyl alcohol determined above, the amount of consumed fluorine-containing alkyl alcohol was calculated (Charged fluorine-containing alkyl alcohol (4.5 mole) - unreacted fluorine-containing alkyl alcohol (1.85 mole) = 2.65 mole), and a consumed amount (245 g: 1.85 mole) of fluorine-containing alkyl alcohol was added to the water layer remaining in the solution of reaction product.Step (F) 761 g of the organic layer obtained in the step (C) was subjected to simple distillation, and fluorine-containing ether having purity (GC analysis) of 99.85 % was obtained at yield of 99.8 %.
References:
Daikin Industries, Ltd. EP2305626, 2011, A1 Location in patent:Page/Page column 12-13

116-15-4
170 suppliers
$40.00/25 g
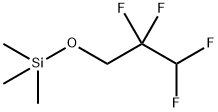
56002-69-8
0 suppliers
inquiry

65064-78-0
32 suppliers
$55.00/1 g