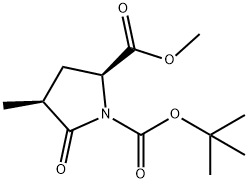
METHYL (2S,4S)-1-(TERT-BUTOXYCARBONYL)-4-METHYLPYROGLUTAMATE synthesis
- Product Name:METHYL (2S,4S)-1-(TERT-BUTOXYCARBONYL)-4-METHYLPYROGLUTAMATE
- CAS Number:196394-48-6
- Molecular formula:C12H19NO5
- Molecular Weight:257.28
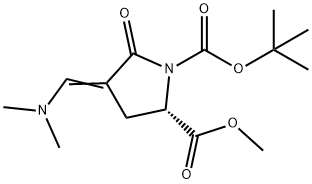
![1,2-Pyrrolidinedicarboxylic acid, 4-[(dimethylamino)methylene]-5-oxo-, 1-(1,1-dimethylethyl) 2-methyl ester, (2S,4E)-](/CAS/20210305/GIF/219844-08-3.gif)
219844-08-3
0 suppliers
inquiry

196394-48-6
15 suppliers
inquiry
Yield:196394-48-6 100%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in isopropyl alcohol at 45; under 2327.23 Torr; for 18 h;
Steps:
1.A
(2S,4S)-1-(1,1-Dimethylethyl)-4-methyl-5-oxo-1,2-pyrrolidinedicarboxylic acid-2-methyl ester (4). A 10-gallon Pfaudler reactor is inerted with nitrogen and charged with ESCAT 142 5% palladium powder on carbon (50% wet, 0.58 Kg wet wt.), intermediate (3) (1.89 Kg, 6.33 mol) and isopropanol (22.4 Kg). The reaction mixture is agitated under a 45-psi hydrogen atmosphere at 45° C. for 18 hrs. The reaction mixture is then cooled to room temperature and filtered though a bed of Celite (0.51 Kg) in a nutsche filter to remove catalyst. The mother liquor is evaporated under reduced pressure to give a thick oil that crystallizes on standing to afford 4 (1.69 Kg, 100%) as a 93:7 diastereomeric mixture. A sample of product mixture is purified by preparative HPLC to give material for analytical data. Anal. Calcd for C12H19NO5: C, 56.0; H, 7.44; N, 5.44. Found C, 55.8; H, 7.31; N, 5.44; MS (ESI+) Expected for C12H19NO5, [M+H] 258.1342. Found 258.1321; 1H NMR (CDCl3, 499.8 MHz) δ=4.44 (m, 1H), 3.72 (s, 3H), 2.60-2.48 (m, 2H), 1.59-1.54 (m, 1H), 1.43 (s, 9H), 1.20 (d, j=6.8 Hz,3H); 13C NMR (CDCl3, 125.7 MHz) δ=175.7, 172.1, 149.5, 83.6, 57.4, 52.5, 37.5, 29.8, 27.9, 16.2. Mp 89.9° C.
References:
US2007/232804,2007,A1 Location in patent:Page/Page column 4; 5
![1,2-Pyrrolidinedicarboxylic acid, 4-[(dimethylamino)methylene]-5-oxo-, 1-(1,1-dimethylethyl) 2-methyl ester, (2S,4E)-](/CAS/20210305/GIF/219844-08-3.gif)
219844-08-3
0 suppliers
inquiry
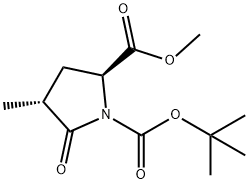
196394-49-7
29 suppliers
$120.00/10mg

196394-48-6
15 suppliers
inquiry
196394-47-5
7 suppliers
inquiry

196394-48-6
15 suppliers
inquiry

196394-47-5
7 suppliers
inquiry

196394-49-7
29 suppliers
$120.00/10mg

196394-48-6
15 suppliers
inquiry
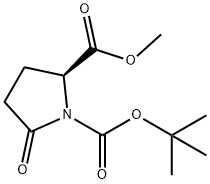
108963-96-8
286 suppliers
$5.00/1g

74-88-4
346 suppliers
$15.00/10g

196394-49-7
29 suppliers
$120.00/10mg

196394-48-6
15 suppliers
inquiry