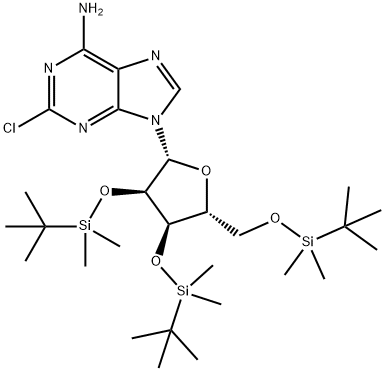
2-Chloro-2',3',5'-tris-O-[(1,1-diMethylethyl)diMethylsilyl]-adenosine synthesis
- Product Name:2-Chloro-2',3',5'-tris-O-[(1,1-diMethylethyl)diMethylsilyl]-adenosine
- CAS Number:195727-26-5
- Molecular formula:C28H54ClN5O4Si3
- Molecular Weight:644.47
146-77-0
435 suppliers
$8.00/250mg
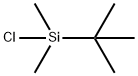
18162-48-6
690 suppliers
$9.00/5g
![2-Chloro-2',3',5'-tris-O-[(1,1-diMethylethyl)diMethylsilyl]-adenosine](/CAS/GIF/195727-26-5.gif)
195727-26-5
14 suppliers
$75.00/50mg
Yield:-
Reaction Conditions:
with 1H-imidazole in tetrahydrofuran;DMF (N,N-dimethyl-formamide);
Steps:
1
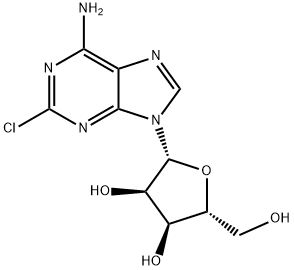
Example 1;-Preparation OF 2-CHLORO-2, 3', 5-TRI-O- (TERT- butyldimethylsilyl) adenosine; [44] To a stirred solution of 2001 g (6.632 mol) of 2-chloroadenosine and 9,997 g (66.32 mol) of t-butyldimethylsilyl chloride in 26L of anhydrous tetrahydrofuran and 16L of DMF was added 9030 g (132.6 mol) of imidazole. Upon disappearance of the starting material (2-chloroadenosine) by TLC, the reaction was concentrated under reduced pressure. The resultant viscous oil was diluted with ethyl acetate (20L) and washed with 2M NAOH (20L), and three times with water (20L each time). The organic layer was dried over magnesium sulfate (1000 g), filtered and the solvent removed under reduced pressure. The residual oil was used in the next step without further purification.
References:
WO2005/33121,2005,A2 Location in patent:Page/Page column 10-11