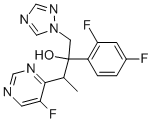
(2R,3S/2S,3R)-2-(2,4-Difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol synthesis
- Product Name:(2R,3S/2S,3R)-2-(2,4-Difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
- CAS Number:182230-43-9
- Molecular formula:C16H14F3N5O
- Molecular Weight:349.31
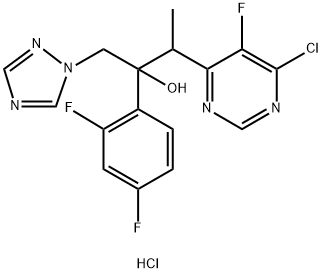
188416-20-8
106 suppliers
$393.00/1g

182230-43-9
113 suppliers
$205.00/2.5mg
Yield:182230-43-9 97.1%
Reaction Conditions:
with palladium 10% on activated carbon;ammsnium formate in ethanol at 30; for 1 h;Inert atmosphere;Large scale;Temperature;
Steps:
1.2-3.2
2) Into the 500L tank, pump 130.4kg of 95% ethanol, add 28.84g of ammonium formate, 55.0kg of Intermediate 2, seal the feeding port, and dissolve the material for 10 minutes.Open the vacuum valve of the tank, evacuate to the gauge pressure -0.06MPa, close the vacuum valve, open the nitrogen valve, charge nitrogen to the gauge pressure +0.06MPa, and close the nitrogen valve.Slowly open the return valve to relieve nitrogen pressure.Open the feeding port, under the protection of small nitrogen flow, pour the thin paste stirred into the stainless steel barrel with 10.0kg 95% ethanol and 0.96kg 10% palladium carbon into the reaction tank, then wash the barrel with 10.0kg 95% ethanol, the lotion Pour them into the tank together, seal the feeding port, close the nitrogen valve, and close the return valve.Slowly open the vacuum valve of the reaction tank, evacuate to the gauge pressure -0.06MPa, close the vacuum valve, open the nitrogen valve, slowly charge nitrogen to the gauge pressure +0.06Mpa, close the nitrogen valve, and observe for 1 minute to confirm that there is no leakage; repeat the operation 2 times , and shared nitrogen to replace the air 3 times.Incubate for 1 hour at 30°C.In the state of stirring operation, press filtration with nitrogen gas, after the filtration liquid is cleaned, pass steam to heat to the internal temperature of 60 ° C, and when about one third of the volume is left, 20.0 kg of drinking water is drawn in, and the distillation is continued until no droplets drip. , the distillation is over.110.0 kg of drinking water was pumped in under stirring, heated to an internal temperature of 40° C. in a water bath, and kept for 3 hours.The filter cake was washed with 11.0kg of drinking water (25°C).After dumping, delay the shutdown for 30 minutes.The filter cake was dried at 60°C for 8 hours to obtain 35.83 kg of hydride (intermediate 3), yield 97.1%,
References:
CN114920729,2022,A Location in patent:Paragraph 0013-0025
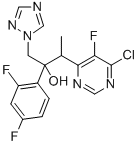
188416-35-5
184 suppliers
$145.00/1g

182230-43-9
113 suppliers
$205.00/2.5mg
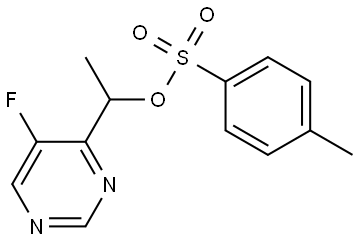
1289559-67-6
0 suppliers
inquiry
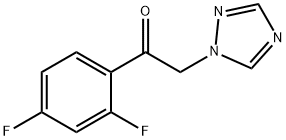
86404-63-9
423 suppliers
$19.00/25g

182230-43-9
113 suppliers
$205.00/2.5mg
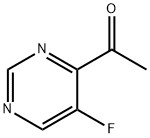
1289559-75-6
2 suppliers
inquiry

182230-43-9
113 suppliers
$205.00/2.5mg
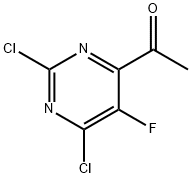
1289559-65-4
5 suppliers
inquiry

182230-43-9
113 suppliers
$205.00/2.5mg