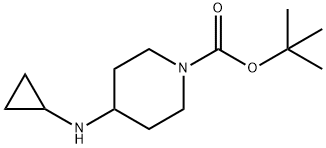
1-TERT-BUTOXYCARBONYL-4-(CYCLOPROPYLAMINO)PIPERIDINE synthesis
- Product Name:1-TERT-BUTOXYCARBONYL-4-(CYCLOPROPYLAMINO)PIPERIDINE
- CAS Number:179557-01-8
- Molecular formula:C13H24N2O2
- Molecular Weight:240.34
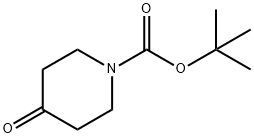
79099-07-3
543 suppliers
$5.00/5g
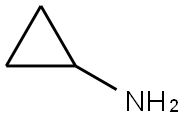
765-30-0
487 suppliers
$10.00/25g

179557-01-8
69 suppliers
$45.00/50mg
Yield:179557-01-8 88%
Reaction Conditions:
Stage #1: N-tert-butyloxycarbonylpiperidin-4-one;Cyclopropylaminewith triethylamine;zinc(II) chloride in methanol at 60; for 7 h;
Stage #2: with sodium cyanoborohydride in methanol at 25; for 17 h;
Steps:
To Intermediate 1, tert-butyl 4-oxopiperidine-1-carboxylate (5.0 g, 25.1 mmol) in MeOH (40mL) was added Intermediate 7, cyclopropanamine (1.4 g, 25.1 mmol), Et3N (10.0 mL, 75.3mmol) and ZnCI2 (0.3 g, 2.5 mmol). The reaction mixture was stirred at 60 °C for 7 h, then NaBH3CN (4.8 g, 75.3 mmol) was added portionwise. The resulting reaction mixture was stirred at 25 °C for 17 h. The solvents were removed in vacuo, and the residue was partitioned between H20 (250 mL) and EtOAc (200 mL). The aqueous layer was extracted further withEtOAc (2 x 200 mL), the combined organic layers were dried (Na2SO4) and the solvent was removed in vacuo. The residue was purified by column chromatography (normal basic activated alumina, 10 % to 30 % EtOAc in hexane) to give Intermediate 11, tert-butyl 4- (cyclopropylamino)piperidine-1-carboxylate (5.3 g, 88 %) as a gum.The data for Intermediate 11 are in Table 2.
References:
WO2017/21730,2017,A1 Location in patent:Page/Page column 46
41979-39-9
115 suppliers
$15.00/10mg

179557-01-8
69 suppliers
$45.00/50mg
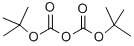
24424-99-5
833 suppliers
$13.50/25G

179557-01-8
69 suppliers
$45.00/50mg