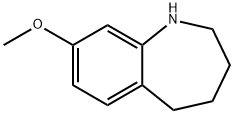
8-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[B]AZEPINE HYDROCHLORIDE synthesis
- Product Name:8-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[B]AZEPINE HYDROCHLORIDE
- CAS Number:17422-43-4
- Molecular formula:C11H15NO
- Molecular Weight:177.24
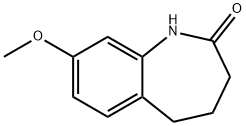
22246-83-9
30 suppliers
inquiry
![8-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[B]AZEPINE HYDROCHLORIDE](/CAS/GIF/17422-43-4.gif)
17422-43-4
28 suppliers
inquiry
Yield:17422-43-4 89%
Reaction Conditions:
Stage #1: 8-methoxy-2,3,4,5-tetrahydro-1H-[1]benzazepin-2-onewith lithium aluminium tetrahydride in tetrahydrofuran; for 4 h;Reflux;
Stage #2: with water;sodium hydroxide in tetrahydrofuran;
Steps:
65 8-methoxy-2,3,4, 5-tetrahydro-1H-1-benzazepine
Reference Example 65 8-methoxy-2,3,4, 5-tetrahydro-1 H-1-benzazepine To a suspension of lithium aluminum hydride (725 mg, 19.1 mmol) in THF (15 mL) was added dropwise a solution of 8-methoxy-1,3,4, 5-tetrahydro-2H-1-benzazepin-2-one (1.46 g, 7.63 mmol) in THF (20 mL) under ice-cooling, and the mixture was refluxed for 4 hr. The reaction mixture was treated by adding dropwise water (0.75 mL), 15% aqueous sodium hydroxide solution (0.75 mL) and water (2.25 mL). The precipitate was filtered and the obtained solid was washed with ethyl acetate. The filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography to give the title compound (1.20 g, yield 89%) as an oil. 1H-NMR (CDCl3) δ: 1.55 - 1.69 (2H, m), 1.72 - 1.89 (2H, m), 2.64 - 2.78 (2H, m), 2.99 - 3.12 (2H, m), 3.75 (4H, s), 6.30 (1H, d, J = 2.6 Hz), 6.38 (1H, dd, J = 8.2, 2.5 Hz), 6.99 (1H, d, J = 8.1 Hz).
References:
EP2298731,2011,A1 Location in patent:Page/Page column 62-63
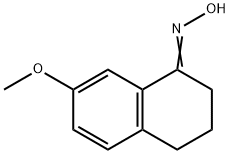
20175-97-7
13 suppliers
inquiry
![8-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[B]AZEPINE HYDROCHLORIDE](/CAS/GIF/17422-43-4.gif)
17422-43-4
28 suppliers
inquiry

6836-19-7
505 suppliers
$14.00/5g
![8-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[B]AZEPINE HYDROCHLORIDE](/CAS/GIF/17422-43-4.gif)
17422-43-4
28 suppliers
inquiry
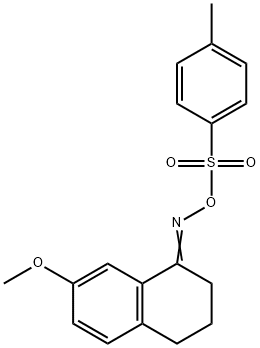
99833-87-1
21 suppliers
$28.60/25MG
![8-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[B]AZEPINE HYDROCHLORIDE](/CAS/GIF/17422-43-4.gif)
17422-43-4
28 suppliers
inquiry