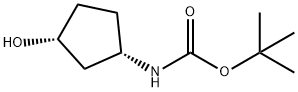
Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:167465-99-8
- Molecular formula:C10H19NO3
- Molecular Weight:201.26
![1-[(3S,1R)-3-N-BOC-aminocyclopentyl]-1-ethanone](/CAS/20180629/GIF/204913-01-9.gif)
204913-01-9
16 suppliers
inquiry
![Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167465-99-8.gif)
167465-99-8
110 suppliers
$35.00/100mg
Yield:167465-99-8 95.3%
Reaction Conditions:
Stage #1: 1-[(3S,1R)-3-N-BOC-aminocyclopentyl]-1-ethanonewith carbamide peroxide;trifluoroacetic anhydride in tetrahydrofuran at 0; for 7 h;Inert atmosphere;
Stage #2: with water;sodium hydroxide in ethanol at 10 - 20; for 5 h;Reagent/catalyst;Temperature;
Steps:
1.2; 2.2; 3.2; 4.2; 5.2
(1) Under a nitrogen atmosphere, 600 mL of tetrahydrofuran was introduced into the reaction flask.Turn on the stirring device and put 60.1g of carbamide peroxide, 60g of compound I,The control temperature was 0 ° C, and 138.5 g of trifluoroacetic anhydride was added dropwise to the obtained mixture.After the addition is completed, the oxidation reaction is carried out for 7 hours;After the end of the oxidation reaction by GC, the pH of the obtained system was adjusted to 7.5 with a sodium carbonate solution having a mass concentration of 30%, the temperature was controlled at <10 ° C, stirring was continued for 10 min, and then the layer was allowed to stand, and the obtained aqueous phase was subjected to ethyl acetate. extraction,The organic phase was washed with 180 mL of a 5% solution of sodium thiosulfate and washed with aq.The resulting concentrate is a mixture of Compound II and Compound II' (GC detected the purity of the concentrate to be 88.0% based on the total purity of Compound II and Compound II'); (2) transferring a mixture of the compound II and the compound II' to a reaction flask,Add 300mL of ethanol, turn on the stirring device, and control the temperature to 10 °c.Adding lithium hydroxide aqueous solution dropwise(Formulated by 19.4g of lithium hydroxide dissolved in 180mL of water),After the completion of the dropwise addition, the hydrolysis reaction is carried out at room temperature for 5 h;After the completion of the GC monitoring reaction, the obtained system was allowed to stand for stratification, and the obtained aqueous phase was extracted with 240 mL of ethyl acetate, and the extraction was repeated once.The organic phase was combined, and the obtained organic phase was washed with brine (180 mL).After filtration, the obtained organic phase was concentrated under reduced pressure.The obtained concentration was concentrated to obtain Compound III (the purity of the concentrate was determined by GC to be 92.0%, and the yield was 94.0%);
References:
CN108774145,2018,A Location in patent:Paragraph 0080-0082; 0085; 0087; 0089; 0091; 0093; 0095
![Carbamic acid, [(1S,3R)-3-(acetyloxy)cyclopentyl]-, 1,1-dimethylethyl ester](/CAS/20180702/GIF/225641-82-7.gif)
225641-82-7
9 suppliers
inquiry
![Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167465-99-8.gif)
167465-99-8
110 suppliers
$35.00/100mg
![Tert-Butyl 2-Oxa-3-Azabicyclo[2.2.1]Hept-5-Ene-3-Carboxylate](/CAS/GIF/CB73144508.gif)
99027-90-4
38 suppliers
$17.00/100mg
![Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167465-99-8.gif)
167465-99-8
110 suppliers
$35.00/100mg

24424-99-5
852 suppliers
$13.50/25G
1110772-05-8
93 suppliers
$28.00/10mg
![Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167465-99-8.gif)
167465-99-8
110 suppliers
$35.00/100mg

24424-99-5
852 suppliers
$13.50/25G
![Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167465-99-8.gif)
167465-99-8
110 suppliers
$35.00/100mg