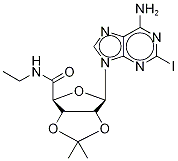
2-IODO-5'-ETHYLCARBOXAMIDO-2',3'-O-ISOPROPYLIDINEADENOSINE synthesis
- Product Name:2-IODO-5'-ETHYLCARBOXAMIDO-2',3'-O-ISOPROPYLIDINEADENOSINE
- CAS Number:162936-24-5
- Molecular formula:C15H19IN6O4
- Molecular Weight:474.25
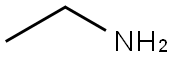
75-04-7
405 suppliers
$10.00/20mg
141018-26-0
19 suppliers
$85.00/5mg

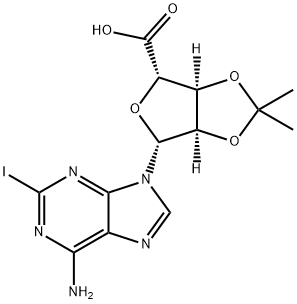
162936-24-5
19 suppliers
$62.50/5 mg
Yield: 71%
Reaction Conditions:
Stage #1:(3aS,4S,6R,6aR)-6-(6-amino-2-iodo-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxylic acid with N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline in ethanol;dichloromethane at 20; for 24 h;
Stage #2:ethylamine in ethanol;dichloromethane at 20; for 24 h;
Steps:
A.A3; 3 SCHEME A; step A3; Example 3: Synthesis of N-ethyl-1'-deoxy-1'-(6-amino-2-iodo-9H-purin-9-yl)-2',3'-O-isopropylidene-β-D-ribofuranuronamide (Compound 4)
To a solution of Compound 3 (8. 0 g, 17.9 mmol) in 50% ETHANOL/CH2CL2 (160 mL) was added 2-ETHOXY-1-ETHOCYCARBONYL-1, 2-dihydroquinoline (EEDQ) (4.65 g, 18. 8 mmol) as a single portion. The mixture was stirred at room temperature for 24 hours. Ethanol (80 ML) was added and ethylamine gas (-45 g) bubbled through the reaction solution over 4 hours. The reaction was stirred at room temperature for 20 hours after which the solvents were evaporated. The resulting solids were dissolved in CH2C12 and washed successively with 0.1 M HCl and 1M NA2C03. The organics were dried (NA2S04), filtered, and evaporated to afford Compound 4 as a pale yellow solid which was recrystallized from CH2CL2/HEXANES to afford Compound 4 (6.0 g, 71%) as a white solid, m. p. 204-206°C ; 1H-NMR (600 MHz, DMSO- d6): 0.68 (t, 3H), 1.32 (s, 3H), 1.52 (s, 3H), 2.81 (m, 1H), 2.91 (m, 1H), 4.53 (s, 1H), 5.33 (m, 1H), 5. 36 (m, 1H), 6.27 (s, 1H), 7.43 (t, 1H), 7. 68 (s, 2H), 8.16 (s, 1H) ; 13C-NMR (150 MHz, DMSO-d6): 14.07, 25.06, 26.61, 33.07, 83.15, 83.35, 112.77, 118.71, 120.68, 139.99, 149.31, 155.81, 167.92. LRMS (ES): M/Z = 475.0 (M+H, 100%).
References:
BRISTOL-MEYERS SQUIBB PHARMA COMPANY WO2004/104017, 2004, A2 Location in patent:Page 18; 32
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

162936-24-5
19 suppliers
$62.50/5 mg