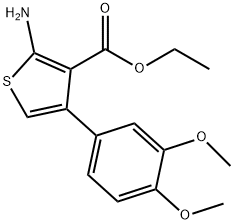
2-AMINO-4-(3,4-DIMETHOXY-PHENYL)-THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:2-AMINO-4-(3,4-DIMETHOXY-PHENYL)-THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:15854-12-3
- Molecular formula:C15H17NO4S
- Molecular Weight:307.36
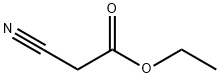
105-56-6
439 suppliers
$10.00/5ml
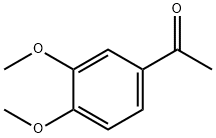
1131-62-0
319 suppliers
$14.00/5g

15854-12-3
20 suppliers
$126.00/500mg
Yield:15854-12-3 42%
Reaction Conditions:
Stage #1: ethyl 2-cyanoacetate;1-(3,4-dimethoxyphenyl)ethanonewith ammonium acetate;acetic acid in toluene;Dean-Stark;Reflux;Gewald Aminoheterocycles Synthesis;
Stage #2: with sulfur;diethylamine in ethanol at 50; for 3 h;Gewald Aminoheterocycles Synthesis;
Steps:
Method A. Synthesis of alkyl 2-amino-4-arylthiophene-3-carboxylates
General procedure: To a solution ofarylacetophenone (30 mmol) in benzene or toluene (10 mL) was added ethylcyanoacetate or malononitrile (66 mmol), ammonium acetate (15 mmol) and acetic acid (60 mmol) and the reaction mixture was refluxed for 18 - 48h using a Dean Stark apparatus. After completion of reaction, the reaction mixture was cooled to room temperature, quenched with water (200 ml) and extracted with ethyl acetate (100ml x 2). The organic phase was dried over sodium sulphate, concentrated under reduced pressure and the residue was dissolved in ethanol (120 mL). Sulphur powder (40 mmol) and diethylamine (26 mmol) were added to the solution, which was heated at 50°C for 3h. The hot solution was then filtered to remove unreacted sulphur. The resulting filtrate was concentrated to give crude product, which was purified by flash chromatography to afford the corresponding alkyl 2-amino-4-arylthiophene-3-carboxylate or 3-cyano-2-amino-4-arylthiophene.
References:
Titchenell, Paul M.;Hollis Showalter;Pons, Jean-Fran?ois;Barber, Alistair J.;Jin, Yafei;Antonetti, David A. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 10,p. 3034 - 3038] Location in patent:supporting information