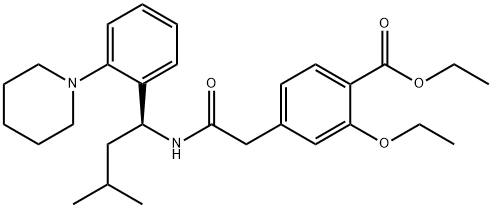
(+)-2-Ethoxy-4-(N-3-Methyl-1(S)-(2-(1-Piperidinyl)Phenyl)-Butyl)Carbamoylmethyl) synthesis
- Product Name:(+)-2-Ethoxy-4-(N-3-Methyl-1(S)-(2-(1-Piperidinyl)Phenyl)-Butyl)Carbamoylmethyl)
- CAS Number:147770-06-7
- Molecular formula:C29H40N2O4
- Molecular Weight:480.64
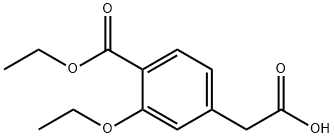
99469-99-5
313 suppliers
$9.00/1g
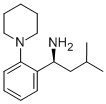
147769-93-5
193 suppliers
$34.00/250mg

147770-06-7
102 suppliers
$50.00/5 mg
Yield: 93%
Reaction Conditions:
Stage #1:2-(3-ethoxy-4-(ethoxycarbonyl)phenyl) acetic acid with 1,1'-carbonyldiimidazole in dichloromethane
Stage #2:(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butan-1-amine in dichloromethaneProduct distribution / selectivity;
Steps:
11 Example 11
Example 11 (S)(+)-2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonyl-methyl]-benzoic acid ethyl ester 20.77 g (82.4 mmol) of 2-(3-Ethoxy-4-(ethoxycarbonyl)phenyl)-acetic acid were slowly added to a solution of N,N'-carbonyldiimidazole (13.36 g, 82.4 mmol, 97%) in dichloromethane (240 ml) at room temperature. After activation for 1.5h at 20-25°C a solution of (S)-3-methyl-1-(2-(piperidin-1-yl)phenyl)butan-1-amine (19.70 g, 80.0 mmol) in dichloromethane (80 ml) was added dropwise and the resulting mixture was stirred for 2h. The reaction mixture was quenched by adding water (2 x 160 ml). The organic phase was then evaporated under reduced pressure. The yield of the crude repaglinide ester product of formula IV was 35.7 g (93%; HPLC: 97.58%).
References:
Krka Tovarna Zdravil, D.D., Novo Mesto EP2177221, 2010, A1 Location in patent:Page/Page column 13
34595-26-1
84 suppliers
$11.00/500mg

147770-06-7
102 suppliers
$50.00/5 mg
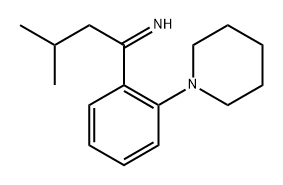
147769-96-8
3 suppliers
inquiry

147770-06-7
102 suppliers
$50.00/5 mg
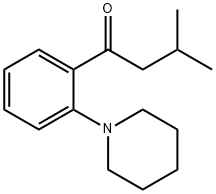
147770-03-4
7 suppliers
inquiry

147770-06-7
102 suppliers
$50.00/5 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

147770-06-7
102 suppliers
$50.00/5 mg