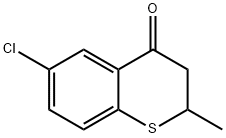
6-CHLORO-2-METHYL-3,4-DIHYDRO-2H-1-BENZOTHIIN-4-ONE synthesis
- Product Name:6-CHLORO-2-METHYL-3,4-DIHYDRO-2H-1-BENZOTHIIN-4-ONE
- CAS Number:147713-35-7
- Molecular formula:C10H9ClOS
- Molecular Weight:212.7
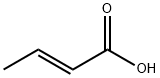
107-93-7
311 suppliers
$11.19/100G
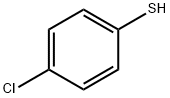
106-54-7
247 suppliers
$10.00/5g

147713-35-7
10 suppliers
inquiry
Yield:147713-35-7 105 g
Reaction Conditions:
Stage #1: (E)-but-2-enoic acid;p-Chlorothiophenolwith triethylamine in chlorobenzene at 80; for 6 h;
Stage #2: with thionyl chloride in N,N-dimethyl acetamide;chlorobenzene at 50; for 1 h;
Stage #3: with aluminum (III) chloride in N,N-dimethyl acetamide;chlorobenzene at 8; for 4 h;
Steps:
Synthesis of A-2
[0472] Crotonic acid (59.3 g) was added a suspension of 4-chlorobenzenethiol (100 g) in chlorobenzene (100 mL), triethylamine (28.9 mL) was added dropwise thereto, and a reaction was carried out at 80° C. for 6 hours. After cooling to 50° C., N,N-dimethylacetamide (0.1 mL) was added to the reaction mixture, thionyl chloride (50.2 mL) was added dropwise thereto, and a reaction was carried out for 1 hour. The reaction mixture was added dropwise under ice cooling to a suspension of aluminum chloride (96.8 g) in chlorobenzene (200 mL) that had been prepared in advance, and a reaction was carried out at less than 8° C. for 4 hours. The reaction mixture was poured into 1,000 g of ice, and the organic layer was separated, then washed with 500 mL of water, and concentrated, thus giving crude crystals. The crude crystals were recrystallized from methanol (150 mL), filtered, and washed with cold methanol, thus giving a ketone compound (105 g).
References:
US2013/171415,2013,A1 Location in patent:Paragraph 0472
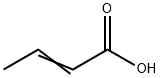
3724-65-0
183 suppliers
$1146.05/25G

106-54-7
247 suppliers
$10.00/5g

147713-35-7
10 suppliers
inquiry
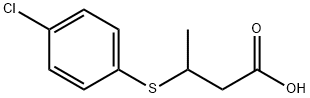
90919-34-9
21 suppliers
$60.00/100mg

147713-35-7
10 suppliers
inquiry