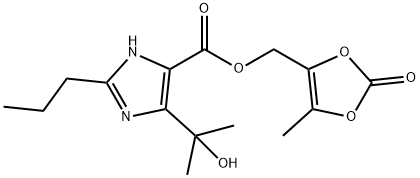
(5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 5-(2-hydroxypropan-2-yl)-2-propyl-1H-imidazole-4-carboxylate synthesis
- Product Name:(5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 5-(2-hydroxypropan-2-yl)-2-propyl-1H-imidazole-4-carboxylate
- CAS Number:144978-05-2
- Molecular formula:C15H20N2O6
- Molecular Weight:324.33
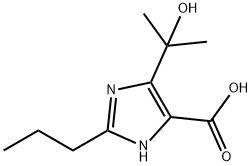
144690-04-0
50 suppliers
inquiry
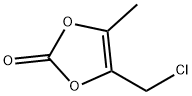
80841-78-7
546 suppliers
$10.00/250mg

144978-05-2
22 suppliers
inquiry
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl acetamide;ethyl acetate;
Steps:
31 (5-Methyl-2-oxo-1,3-dioxolen-4-yl)methyl 4-(1-hydroxy-1-methylethyl)-2-propylimidazole-5-carboxylate
PREPARATION 31 (5-Methyl-2-oxo-1,3-dioxolen-4-yl)methyl 4-(1-hydroxy-1-methylethyl)-2-propylimidazole-5-carboxylate 1.76 ml of N,N-diisopropylethylamine were added to a suspension of 1.06 g of 4-(1-hydroxy-1-methylethyl)-2-propylimidazole-5-carboxylic acid [prepared as described in Preparation 22(i)] in 10 ml of N,N-dimethylacetamide, and the resulting mixture was stirred at room temperature for 10 minutes, after which 1.12 g of (5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl chloride was added, and the mixture was stirred at 60° C. for 4 hours. At the end of this time, the reaction mixture was mixed with ethyl acetate and water. The ethyl acetate layer was separated and concentrated by evaporation under reduced pressure, and the concentrate was purified by column chromatography through silica gel, using a 1:15 by volume mixture of methanol and methylene chloride as the eluent, to give 1.14 g of the title compound as a viscous oil. Nuclear Magnetic Resonance Spectrum (CDCl3), δ ppm: 0.94 (3H, triplet, J=7.5 Hz); 1.62 (6H, singlet); 1.6-1.8 (2H, multiplet); 2.19 (3H, singlet); 2.67 (2H, triplet. J=8 Hz); 5.03 (2H, singlet).
References:
US5616599,1997,A