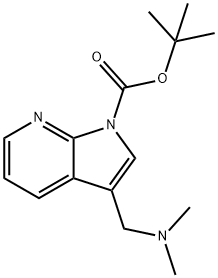
3-Dimethylaminomethyl-pyrrolo[2,3-b]pyridine-1-carboxylic acid tert-butyl ester, 1-tert-Butoxycarbonyl-3-[(dimethylamino)methyl]-7-azaindole synthesis
- Product Name:3-Dimethylaminomethyl-pyrrolo[2,3-b]pyridine-1-carboxylic acid tert-butyl ester, 1-tert-Butoxycarbonyl-3-[(dimethylamino)methyl]-7-azaindole
- CAS Number:144657-65-8
- Molecular formula:C15H21N3O2
- Molecular Weight:275.35
![1-(2,9-diazabicyclo[4.3.0]nona-2,4,7,10-tetraen-7-yl)-N,N-dimethyl-methanamine](/CAS/GIF/5654-92-2.gif)
5654-92-2
69 suppliers
$30.00/250mg

24424-99-5
835 suppliers
$13.50/25G
![3-Dimethylaminomethyl-pyrrolo[2,3-b]pyridine-1-carboxylic acid tert-butyl ester, 1-tert-Butoxycarbonyl-3-[(dimethylamino)methyl]-7-azaindole](/CAS/GIF/144657-65-8.gif)
144657-65-8
13 suppliers
$148.00/1g
Yield:144657-65-8 96.7%
Reaction Conditions:
Stage #1: 7-azagraminewith sodium hydride in tetrahydrofuran; for 0.166667 h;
Stage #2: di-tert-butyl dicarbonate in tetrahydrofuran at 20;
Steps:
4.1
Step 1: Preparation of3-Dimethylaminomethyl-pyrrolo[2,3-b]pyridine-l-carboxylic acid tert- butyl ester (511); [0112] To dimethyl-(lH-pyrrolo[2,3-b]pyridin-3-ylmethyl)-amine (2, 2.50 g, 14.3 mmol.) in tetrahydrofuran (200.0 mL) was added sodium hydride (0.685 g, 60% in mineral oil, 17.1 mmol). After 10 minutes, di-te^-butyldicarbonate (3.74 g, 17.1 mmol) was added to the reaction. The reaction was stirred al room temperature overnight. The reaction was poured into water and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated and purified by silica gel column chromatography eluting with 30% ethyl acetate in hexane to give as a white solid (511, 3.80 g, 96.7%).
References:
WO2008/63888,2008,A2 Location in patent:Page/Page column 60
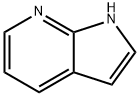
271-63-6
574 suppliers
$9.00/5g
![3-Dimethylaminomethyl-pyrrolo[2,3-b]pyridine-1-carboxylic acid tert-butyl ester, 1-tert-Butoxycarbonyl-3-[(dimethylamino)methyl]-7-azaindole](/CAS/GIF/144657-65-8.gif)
144657-65-8
13 suppliers
$148.00/1g