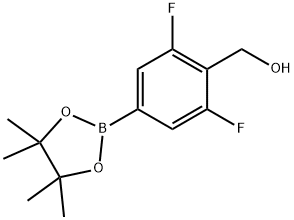
2,6-difluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-benzenemethanol synthesis
- Product Name:2,6-difluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-benzenemethanol
- CAS Number:1417736-43-6
- Molecular formula:C13H17BF2O3
- Molecular Weight:270.08
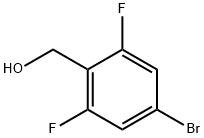
162744-59-4
183 suppliers
$8.00/1g
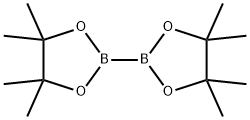
73183-34-3
591 suppliers
$6.00/5g

1417736-43-6
12 suppliers
inquiry
Yield:1417736-43-6 63%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 130; for 1 h;Inert atmosphere;Microwave irradiation;
Steps:
Intermediate 1 A [2,6-Difluoro-4-(4,4,5,5 etramethyl-l ,3,2-dioxaborolan-2-yl)phenyl]methanol
Intermediate 1 A [2,6-Difluoro-4-(4,4,5,5 etramethyl-l ,3,2-dioxaborolan-2-yl)phenyl]methanol (4-Bromo-2,6-difluorophenyl)methanol (1.03 g, 4.62 mmol) was dissolved in dry 1,4-dioxane (10 mL). Argon was bubbled through the solution, then [l ,l'-bis(diphenylphosphino)ferrocene]- dichloropalladium-dichloromethane complex (302 mg, 0.37 mmol, 0.08 eq.), anhydrous potassium acetate (907 mg, 9.24 mmol, 2 eq.) and bis(pinacolato)diboron (1.23 g, 4.85 mmol, 1.05 eq.) were added, and the mixture was heated to 130°C for 1 h in a single-mode microwave device. Upon cooling, the mixture was filtered, and the solvent was removed under reduced pressure. Cyclohexane (200 mL) was added to the residue, and the mixture was vigorously stirred for 30 min. Undissolved material was then removed by filtration, the cyclohexane was distilled off, and the residue was purified by flash chromatography over silica gel (dichloromethane/acetonitrile gradient). Fractions containing the product were combined and evaporated. The title compound crystallized spontaneously as a brownish solid. Yield: 790 mg (63% of th.). GC-MS (method 8): Rt = 5.36 min; MS (EI): m/z (%) = 270.3 (15) [M]+.
References:
WO2013/4551,2013,A1 Location in patent:Page/Page column 33