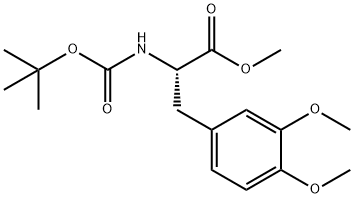
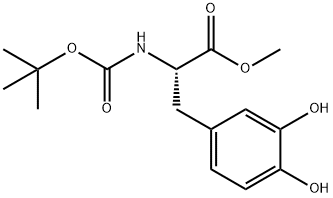
L-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-3-methoxy-O-methyl-, methyl ester synthesis
- Product Name:L-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-3-methoxy-O-methyl-, methyl ester
- CAS Number:138908-56-2
- Molecular formula:C17H25NO6
- Molecular Weight:339.38

24424-99-5
833 suppliers
$13.50/25G
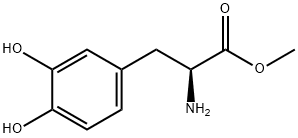
7101-51-1
28 suppliers
$498.06/5MG
![L-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-3-methoxy-O-methyl-, methyl ester](/CAS/20210111/GIF/138908-56-2.gif)
138908-56-2
2 suppliers
inquiry
Yield: 94.4%
Reaction Conditions:
Stage #1:L-DOPA methyl ester with thionyl chloride in methanol at 50; for 3 h;Inert atmosphere;Cooling with ice;
Stage #2:di-tert-butyl dicarbonate with sodium hydrogencarbonate in tetrahydrofuran at 20; for 1 h;Cooling with ice;
Stage #3: with potassium carbonate;dimethyl sulfate in acetone at 20;Reflux;
Steps:
1.1 (1) Preparation(S) -2-((tert-Butoxycarbonyl) amino) -3- (3,4-dimethoxyphenyl) propionic acid methyl ester(A-A)
Take L-dopa (10.0g, 50.71mmol) and dissolve it in 200mL of dry methanol solution.After N2 gas was repeatedly replaced three times, after 15 minutes of ice-salt bath,Dichlorosulfoxide (36.78 mL, 507.13 mmol) was added dropwise, and the ice-salt bath was for 1 h.After warming to 50 ° C for 2h. The excess methanol solution was evaporated under reduced pressure,A large amount of white solid was obtained, and the obtained solid was directly used in the next reaction.The above solid was dissolved in 125 mL of tetrahydrofuran, and a saturated sodium bicarbonate solution was added until the solution became alkaline. After 15 min of ice-salt bath, di-tert-butyl dicarbonate (12.17 g, 55.78 mmol) was slowly added dropwise, and the reaction was carried out at room temperature for 1 h. The organic solvent was evaporated to dryness, and the residue was dissolved in dichloromethane, washed with saturated sodium chloride three times, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain a yellow oily liquid, which was used for the next reaction.The above liquid was dissolved in 450 mL of acetone solution, potassium carbonate (70.01 g, 507.13 mmol) was added to react at room temperature for 20 minutes, and then dimethyl sulfate (14.42 mL, 152.13 mmol) was added to reflux overnight. Filter through diatomaceous earth, evaporate to dryness under reduced pressure, dilute the residue with CH2Cl2, and use water in turn.It was washed with a saturated sodium chloride solution, and the filtrate was dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation.Silica gel column chromatography (V petroleum ether / V ethyl acetate / V dichloromethane = 3/1/1),A-A was obtained as a light yellow solid (16.02 g, yield 94.40%).
References:
South China Agricultural University;Song Gaopeng;Liao Yixian;Li Hui;Li Sumei;Chen Jianxin CN110724102, 2020, A Location in patent:Paragraph 0054; 0056; 0059-0062
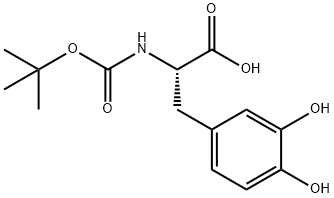
30033-24-0
49 suppliers
$146.66/0.5g
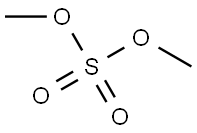
77-78-1
304 suppliers
$19.69/1ml
![L-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-3-methoxy-O-methyl-, methyl ester](/CAS/20210111/GIF/138908-56-2.gif)
138908-56-2
2 suppliers
inquiry
37169-36-1
30 suppliers
$8.00/250mg

74-88-4
347 suppliers
$15.00/10g
![L-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-3-methoxy-O-methyl-, methyl ester](/CAS/20210111/GIF/138908-56-2.gif)
138908-56-2
2 suppliers
inquiry
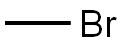
74-83-9
2 suppliers
$24.10/48624

37169-36-1
30 suppliers
$8.00/250mg
![L-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-3-methoxy-O-methyl-, methyl ester](/CAS/20210111/GIF/138908-56-2.gif)
138908-56-2
2 suppliers
inquiry

37169-36-1
30 suppliers
$8.00/250mg

77-78-1
304 suppliers
$19.69/1ml
![L-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-3-methoxy-O-methyl-, methyl ester](/CAS/20210111/GIF/138908-56-2.gif)
138908-56-2
2 suppliers
inquiry