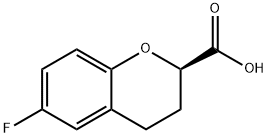
(R)-6-Fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylicacid synthesis
- Product Name:(R)-6-Fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylicacid
- CAS Number:129101-37-7
- Molecular formula:C10H9FO3
- Molecular Weight:196.18
129050-25-5
4 suppliers
inquiry

129101-37-7
69 suppliers
$55.00/100mg
Yield: 96%
Reaction Conditions:
with lithium hydroxide in tetrahydrofuran;methanol;water at 20; for 2 h;
Steps:
22 4.2.21. General procedure for the synthesis of 6a-f
General procedure: To a stirred solution of 2a-f in THF (2 mL) and MeOH (1 mL),0.25 M aqueous LiOH (1.05 equiv) was added dropwise at roomtemperature and stirred for 2 h. The reaction mixture was concentratedunder reduced pressure and then acidified with dil HCl. Thecrude product was extracted with ethyl acetate and concentrated.This organic layer was dried over anhydrous MgSO4, filtered, andevaporated under reduced pressure. The concentrate was furtherpurified by crystallization with n-hexane to afford 6a-f. 4.2.22
(R)-6-Fluorochroman-2-carboxylic acid 6a
Compound 6a (89 mg) was obtained according to Section
4.2.21
from 2a (100 mg, 0.47 mmol).
Yield 96%. 1H NMR: δ 6.88-6.80 (m, 2H), 6.75 (dd, 3J = 8.7, 4JH-F = 2.6, 1H), 4.74 (dd, 1H, J = 7.6, 3.5), 2.90-2.75 (m, 2H), 2.33 (m, 1H), 2.18 (m, 1H); 13C NMR: δ 175.8, 157.2 (d, 1JC-F = 237.9), 148.9 (d, 4JC-F = 2.1), 122.3 (d, 3JC-F = 7.5), 117.8 (d, 3JC-F = 8.1), 115.4 (d, 2JC-F = 22.7), 114.5 (d, 2JC-F = 23.2), 73.2, 24.1, 23.5 (d, 4JC-F = 1.1); [α]D20 = -12.6 (c 1.0, DMF) {lit.
9
[α]D20 = -13.4 (c 1.0, DMF)}; ee >99%, Chiralcel ODH column, n-hexane/2-propanol/trifluoroacetic acid = 90:10:0.5, flow rate = 1.0 mL/min, tR = 6.92 (S) and 9.62 min (R). HRMS calcd for C10H9FO3+ [M]+, 196.0530.
Found 196.0531.
References:
Kim, Dong Woon;Maqusood Alam;Lee, Young Hun;Khan, M. Naseer A.;Zhang, Yong;Lee, Yong Sup [Tetrahedron Asymmetry,2015,vol. 26,# 17,art. no. 59342,p. 912 - 917]