
Carbonic acid 5-[[(1,4-dihydro-4-oxo-3-quinolinyl)carbonyl]amino]-2,4-bis(1,1-dimethylethyl)phenyl methyl ester synthesis
- Product Name:Carbonic acid 5-[[(1,4-dihydro-4-oxo-3-quinolinyl)carbonyl]amino]-2,4-bis(1,1-dimethylethyl)phenyl methyl ester
- CAS Number:1246213-45-5
- Molecular formula:C26H30N2O5
- Molecular Weight:450.53
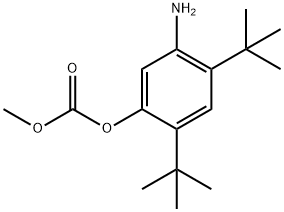
1182822-31-6
82 suppliers
inquiry
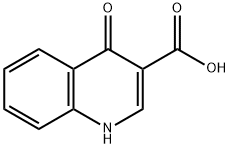
13721-01-2
245 suppliers
$11.00/250mg
![Carbonic acid 5-[[(1,4-dihydro-4-oxo-3-quinolinyl)carbonyl]amino]-2,4-bis(1,1-dimethylethyl)phenyl methyl ester](/CAS/20150408/GIF/1246213-45-5.gif)
1246213-45-5
26 suppliers
inquiry
Yield:1246213-45-5 67%
Reaction Conditions:
Stage #1: 1,4-dihydro-4-oxo-quinoline-3-carboxylic acid esterwith N-ethyl-N,N-diisopropylamine;O-(7-azabenzotriazol-1-yl)-n,n,n',n'-tetramethyluronium hexafluoro-phosphate in N,N-dimethyl-formamide at 25; for 0.333333 h;
Stage #2: 5-amino-2,4-di-tert-butylphenyl methyl carbonate in N,N-dimethyl-formamide at 70; for 2 h;
Steps:
7 Synthesis of 2,4-di-tert-butyl-5-(4-oxo-1,4-dihydroquinoline-3-carboxamido)phenyl methyl carbonate
To a solution of 4-oxo-1,4-dihydroquinoline-3-carboxylic acid (23.5 g, 121mmol) in DMF (400 mL) at 25°C were added HATU (57.2 g, 150 mmol) and DIEA (23.91 g, 185 mmol). The reaction was stirred for 20 min and 5 -amino-2, 4-di-tert-butylphenyl methyl carbonate (38 g, 116 mmol) was added. The reaction was stirred at 70 °C for 2 h, allowed to cool to 25 °C and poured into water (100 mL). The mixture was filtered, and the filtrate concentrated in vacuo. The residue was triturated with petroleum ether/ethyl acetate 3:1 (80 mL) to give the title compound as an off-white solid. Yield 40 g (67%).1H NMR (400 MHz, DMSO-d6): δ 12.00 (s, 1H), 8.88-8.85 (m, 1H), 8.35-8.33 (m, 1H), 7.82-7.79 (m, 1H), 7.76-7.74 (m, 1H), 7.59 (s, 1H), 7.54-7.52 (m, 1H), 7.39 (s, 1H), 4.80 (s, 1H), 3.85-3.79 (m, 3H), 1.46 (s, 9H), 1.32 (s, 9H). m/z: [ESI+]785.2 (M+H)+, (C20H30N2O5).
References:
WO2022/148822,2022,A1 Location in patent:Paragraph 00572-00574
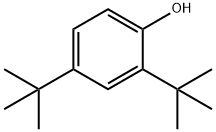
96-76-4
381 suppliers
$6.00/25g
![Carbonic acid 5-[[(1,4-dihydro-4-oxo-3-quinolinyl)carbonyl]amino]-2,4-bis(1,1-dimethylethyl)phenyl methyl ester](/CAS/20150408/GIF/1246213-45-5.gif)
1246213-45-5
26 suppliers
inquiry
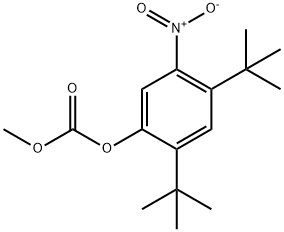
873055-55-1
91 suppliers
$60.00/50mg
![Carbonic acid 5-[[(1,4-dihydro-4-oxo-3-quinolinyl)carbonyl]amino]-2,4-bis(1,1-dimethylethyl)phenyl methyl ester](/CAS/20150408/GIF/1246213-45-5.gif)
1246213-45-5
26 suppliers
inquiry
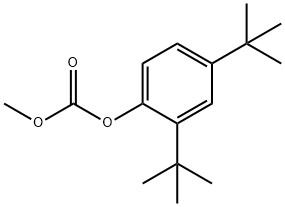
873055-54-0
19 suppliers
inquiry
![Carbonic acid 5-[[(1,4-dihydro-4-oxo-3-quinolinyl)carbonyl]amino]-2,4-bis(1,1-dimethylethyl)phenyl methyl ester](/CAS/20150408/GIF/1246213-45-5.gif)
1246213-45-5
26 suppliers
inquiry
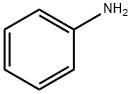
62-53-3
658 suppliers
$10.00/1g
![Carbonic acid 5-[[(1,4-dihydro-4-oxo-3-quinolinyl)carbonyl]amino]-2,4-bis(1,1-dimethylethyl)phenyl methyl ester](/CAS/20150408/GIF/1246213-45-5.gif)
1246213-45-5
26 suppliers
inquiry