
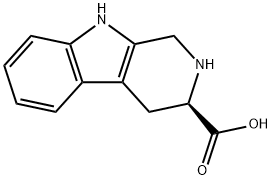
BOC-D-1,2,3,4-TETRAHYDRONORHARMAN-3-CARBOXYLIC ACID synthesis
- Product Name:BOC-D-1,2,3,4-TETRAHYDRONORHARMAN-3-CARBOXYLIC ACID
- CAS Number:123910-26-9
- Molecular formula:C17H20N2O4
- Molecular Weight:316.35

24424-99-5
852 suppliers
$13.50/25G
72002-54-1
60 suppliers
$50.00/1G

123910-26-9
105 suppliers
$18.00/0.25 / G
Yield:-
Reaction Conditions:
with potassium carbonate in tetrahydrofuran;water;acetonitrile
Steps:
1.F N-[(3R)-1,2,3,4-Tetrahydro-9H-pyrido3,4-b1indole-3-carboxy]-L-leucyl-L-aspartic acid amide.
F. 8.73 g di-t-butyl dicarbonate and 8.65 g (3R)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic acid are dissolved together in 150 ml tetrahydrofuran and 5.53 g potassium carbonate is added along with 150 ml of water. The mixture is stirred vigorously overnight. The mixture is evaporated in vacuo to remove most of the THF and the aqueous residue is acidified with 1N hydrochloric acid. This is extracted with ethyl acetate and the organic solution washed with brine, dried over sodium sulfate, filtered, and evaporated. The residue is evaporated from acetonitrile to give (3R)-2-tert-butoxycarbonyl-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic acid.
References:
Rhone-Poulenc Rorer Pharmaceuticals Inc. US5162336, 1992, A