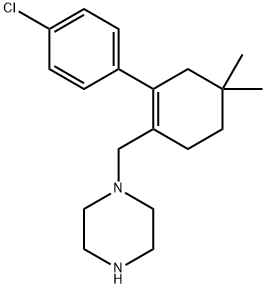
1-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazine synthesis
- Product Name:1-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazine
- CAS Number:1228780-72-0
- Molecular formula:C19H27ClN2
- Molecular Weight:318.88
![4'-chloro-5,5-diMethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-carbaldehyde](/CAS/20150408/GIF/1228837-05-5.gif)
1228837-05-5
102 suppliers
inquiry
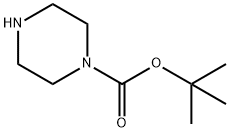
57260-71-6
735 suppliers
$5.00/5g
![1-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazine](/CAS/GIF/1228780-72-0.gif)
1228780-72-0
181 suppliers
$20.00/100mg
Yield:1228780-72-0 80.1%
Reaction Conditions:
Stage #1:4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-carbaldehyde;1-t-Butoxycarbonylpiperazine with sodium tris(acetoxy)borohydride in tetrahydrofuran;toluene at 20; for 8 h;
Stage #2: with hydrogenchloride in water;isopropyl alcohol at 70; for 5 h;
Steps:
Step 3) Synthesis of 1-((4'-Chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-bisphenyl]-2-yl)methyl Yl) piperazine(I-A4)
I-A3 (28.9g, 0.116mol),N-Bocpiperazine (70g, 0.3mol) were dissolved in 400mL oftoluene and tetrahydrofuran.Mix thesolution (V:V=1:1), slowly add sodium triacetoxyborohydride (80.1g, 0.3mol)under stirring,and react at room temperatureShould be 8 hours.Add100mL ofsaturatedNaClaqueous solution toquench the reaction, add 100mL oftoluene, stir for 1 hour, extract and separate the organicFloor.The organic layer was sequentially used with 10% acetic acid aqueous solution (100mL X3), 5%NaHCO3 aqueous solution (100mL X3), saturatedNaClwaterThe solution (100mL X3) was washed, the organic layer was dried over anhydrous sodium sulfate, and then concentrated under reduced pressure. The crude product was subjected to a toluene/acetonitrile system (V:V= 1:4) Recrystallized to obtain 37.1g ofwhite solid.LC-MS m/z: 419.1 [M+H]+. The above product was dissolved in 300mL ofisopropanol, and 10,0g(0.3mol) of concentrated hydrochloric acidwas slowly added.Warm up the reaction solution to70°C, react for 5 hours.After the completion of the reaction, the temperature of the reaction solution was slowly lowered to 0°C, and a solid was precipitatedout afterstirring, and the stirring was continued for 1 hour.PumpFilter, wash the filter cake with a small amount of isopropanol, dissolve the filter cake in 200mL oftolueneafter drying, add 20%fcPO4 aqueous solution (220mL),Warm stirring for 1 hour.The organic layer was separated by extraction. The organic layer was washed with a saturatedaqueousNaClsolution (100mL X2) and then washed with anhydrous sulfurThe filtrate was dried with sodium sulfate, filtered with suction, and evaporated to dryness to obtain 29.1g of white solid. Yield: 80.1%.
References:
UNIV SHENYANG PHARMACEUTICAL;Shenyang Pharmaceutical University;ZHAO LINXIANG;Zhao Linxiang;LIU DAN;Liu Dan;ZHANG ZHENWEI;Zhang Zhenwei;ZHANG JINGYI;Zhang Jingyi;NIU QUN;Niu Qun;JING YONGKUI;Jing Yongkui CN111777626, 2020, A Location in patent:Paragraph 0050; 0097-0099
![tert-butyl 4-((4'-chloro-5,5-diMethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)Methyl)piperazine-1-carboxylate](/CAS/20150408/GIF/1228780-71-9.gif)
1228780-71-9
54 suppliers
inquiry
![1-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazine](/CAS/GIF/1228780-72-0.gif)
1228780-72-0
181 suppliers
$20.00/100mg
110-85-0
563 suppliers
$5.00/5 g
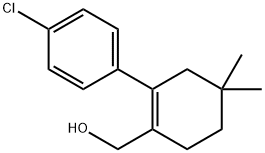
1228780-51-5
47 suppliers
inquiry
![1-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazine](/CAS/GIF/1228780-72-0.gif)
1228780-72-0
181 suppliers
$20.00/100mg
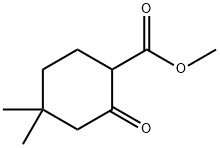
32767-46-7
35 suppliers
$120.00/50mg
![1-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazine](/CAS/GIF/1228780-72-0.gif)
1228780-72-0
181 suppliers
$20.00/100mg
![Methyl 4,4-dimethyl-2-[(trifluoromethylsulfonyl)oxy]cyclohex-1-ene-1-carboxylate](/CAS/20180703/GIF/1228780-46-8.gif)
1228780-46-8
6 suppliers
inquiry
![1-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazine](/CAS/GIF/1228780-72-0.gif)
1228780-72-0
181 suppliers
$20.00/100mg