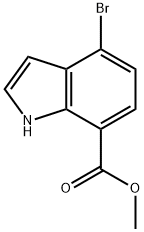
1H-Indole-7-carboxylic acid, 4-broMo-, Methyl ester synthesis
- Product Name:1H-Indole-7-carboxylic acid, 4-broMo-, Methyl ester
- CAS Number:1224724-39-3
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
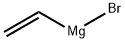
1826-67-1
210 suppliers
$51.29/100ml
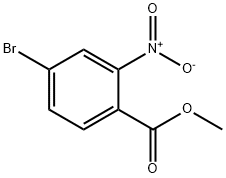
158580-57-5
70 suppliers
$15.00/1g

1224724-39-3
75 suppliers
$65.00/5mg
Yield: 45%
Reaction Conditions:
in tetrahydrofuran;ethanol at -70; for 1 h;Inert atmosphere;
Steps:
10.3 Step 3: Synthesis of compound 10-5
Add compound 10-4 (2.0 g, 7.72 mmol), tetrahydrofuran (20 mL), and replace with nitrogen into a three-necked flask.Cool to -70 in ethanol dry ice bath,Then add ethylmagnesium bromide (28mL, 1mol/L) dropwise,React at this temperature for 1h. The reaction was poured into a saturated ammonium chloride solution, extracted with ethyl acetate three times, the organic phases were combined, and silica gel was added to mix the sample, and then purified by a silica gel column to obtain 680 mg of product 10-5, with a yield of 45%.
References:
CN112608318, 2021, A Location in patent:Paragraph 0233-0234; 0239-0240
1211594-25-0
59 suppliers
$45.00/10mg

74-88-4
347 suppliers
$15.00/10g

1224724-39-3
75 suppliers
$65.00/5mg
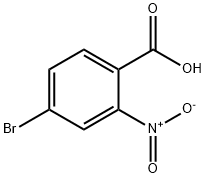
99277-71-1
223 suppliers
$9.00/5g

1224724-39-3
75 suppliers
$65.00/5mg