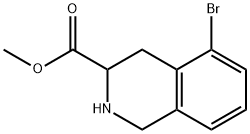
5-BROMO-1,2,3,4-TETRAHYDROISOQUINOLINE-3-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:5-BROMO-1,2,3,4-TETRAHYDROISOQUINOLINE-3-CARBOXYLIC ACID METHYL ESTER
- CAS Number:1219170-24-7
- Molecular formula:C11H12BrNO2
- Molecular Weight:270.12

1219170-24-7
19 suppliers
inquiry
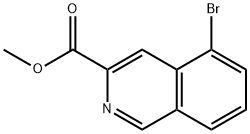
1300033-87-7
3 suppliers
inquiry
Yield:1300033-87-7 91%
Reaction Conditions:
Stage #1: 5-bromo-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid methyl esterwith 2,3-dicyano-5,6-dichloro-p-benzoquinone in tetrahydrofuran; for 18 h;Reflux;
Stage #2: with water;sodium hydroxide in tetrahydrofuran;dichloromethane;
Steps:
17A
Example 17A: methy-5-bromo-isoquinoline-3-carboxylate was prepared using the foilowing procedure describe in US054777252 for methyl-6,7-dimethoxy- isoquinoiine-3-carboxylate: 5-bromo-1 ,2,3,4-tetrahydroisoquinoline-3-carboxylic acid methyl ester (880 mg, 3.26 mmo.) was treated with 2,3-Dichloro-5,6- dicyanobenzoquinone (1.62 mg, 7.19 mmol) in dry tetrahydrofuran (19 ml) at reflux temperature for 18 hours. The cooled dark mixture was filtered and the solid washed with dichloromethane. The filtrate was treated with a 1 M aqueous sodium hydroxide solution and the aqueous layer was extracted twice with dichloromethane. The organic layer was washed with water, brine, dried over sodium sulfate and concentrated to dryness. The residue was purified by flash chromatography over silica gel (gradient cyclohexane/ethyl acetate: 0-100%) to yield methyl 5-bromo- isoquinoiine-3-carboxylaie (793 mg, 91 %) as beige solid.1 H N R : CDCI3 δ (ppm): 9.31 (s, 1 H), 8.90 (s, 1 H), 8.05 (t, J = 9.4 Hz, 2H), 7.61 (t, J = 7.5 Hz, 1 H), 4.09 (s, 3H).
References:
WO2011/51478,2011,A1 Location in patent:Page/Page column 60-61