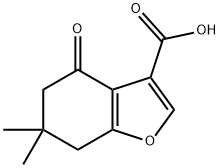
6,6-DIMETHYL-4-OXO-4,5,6,7-TETRAHYDRO-1-BENZOFURAN-3-CARBOXYLIC ACID synthesis
- Product Name:6,6-DIMETHYL-4-OXO-4,5,6,7-TETRAHYDRO-1-BENZOFURAN-3-CARBOXYLIC ACID
- CAS Number:121625-78-3
- Molecular formula:C11H12O4
- Molecular Weight:208.21
Yield:-
Reaction Conditions:
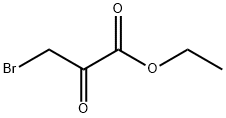
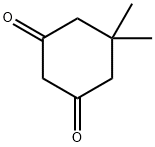
Stage #1: ethyl Bromopyruvate;dimedonewith potassium hydroxide in methanol at 0 - 20; for 16 h;
Stage #2: with methanol;sodium hydroxide;water at 20; for 4 h;
Stage #3: with hydrogenchloride;water in methanol;
Steps:
A
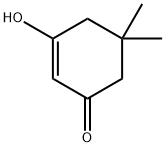
Synthesis of 4-(6,6-Dimethyl-4-oxo-4,5,6 J-tetrahydro-benzofuran-3-ylmethanol (19):oxalylchloride O(1) Step A: 6,6-Dimethyl-4-oxo-4,5,6,7-tetrahydro-benzofuran-3-carboxylic acid (17); 5.5-Dimethyl-1.3-cyclohexandione is dissolved in 15 ml of methanol and cooled to 00C. A solution of KOH (2g, 35.7 mmol) in 15 ml of methanol is added drop wise. A solution of 3- bromo-2-oxo-propionic acid ethyl ester (4.7 ml, 37.5 mmol) in 15 ml of methanol is added drop wise. The mixture is allowed to warm up to room temperature and is stirred for 16h. Then 15 ml of a 45% aqueous sodium hydroxide solution is added. After additional 4 h at room temperature, 25 ml of concentrated HCI is added. Methanol is evaporated slowly under reduced pressure whereas the product precipitates as light crystals. The product is filtrated off and washed with water.
References:
WO2008/101905,2008,A1 Location in patent:Page/Page column 20-21